
Erbi Biosystems Introduces Intensified and Perfusion Microfluidic Bioreactor with Stirred Tank Performance in Just 2 mL Working Volume
Scaled down models are becoming increasingly important to cell line and media development, as well as overall process optimization. These models provide the opportunity to run multiple experiments in parallel at a fraction of the time and cost it would take to run larger scale experiments. In many cases, without scale down models, incredibly informative multi-factorial experiments would not be feasible.
Scaled down, high-cell density N-1 and continuous perfusion cultures are important to data quality, increased staff productivity, and shorter development timelines for bioprocess development. Currently, high-throughput scale-down models are only able to approximate perfusion culture (and at low VCD) with daily media exchanges and only limited sensing and control options. Bench scale stirred tank bioreactors are predominantly used, but they take time to setup and operate. They also require significant amounts of media and other consumables, which can be costly.
In a recent webinar, Erbi Biosystems, presented a small scale, single-use bioreactor platform that provides an automated and robust solution for scale down modeling of intensified and continuous cultures. The webinar, Minimized complexity and robust control with the Erbi Breez™ True Perfusion™ microfluidic bioreactor, presented by Dr. Kevin Lee, Co-founder, discusses Erbi’s first product, the microfluidic reactor named Breez. Due to the novel design, the data presented demonstrates how the Breez can be used to grow and maintain high cell densities of up to 250M cells/mL.
Erbi Breez Microbioreactor Platform
Dr. Lee began the webinar by discussing how, traditionally; perfusion culture has meant stirred tank bioreactors with hollow fiber filters. In these systems, set up and operation is quite involved and requires a significant amount of space, up to three feet of bench space for a single reactor. In addition, the volume of media can be hundreds of liters a week. Maintenance for these systems also requires significant time and effort with higher costs.
He shared that at Erbi, they believe going small can solve most of these issues. With the Breez, their aim was to fit the equivalent of a dozen fully functional, stirred tank bioreactors in the same three-foot space of a single 3L stirred tank bioreactor. Another design objective was for operation to only require a single person for these 12 from start-to-finish with no sacrifice to performance.
A Scaled Down Case Study
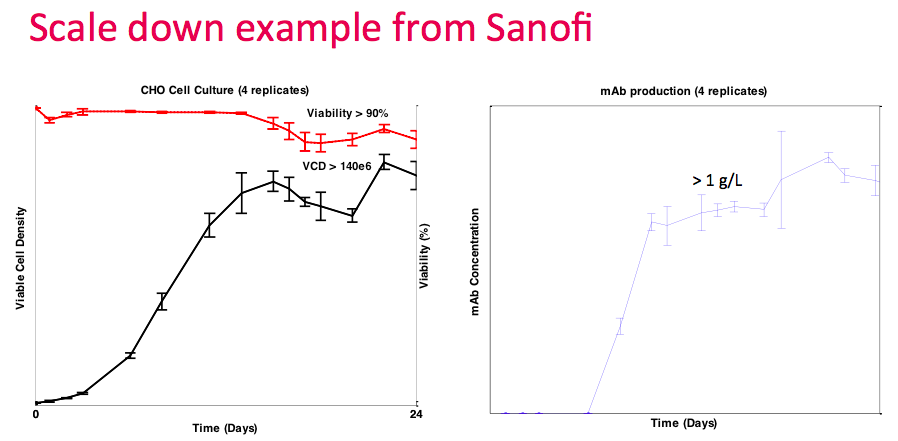
Next Dr. Lee presented data from a collaboration with Sanofi. They demonstrated that with the Breez, they could run multiple perfusion cultures simultaneously and achieve stirred tank performance with cell densities at 140M cells/mL and greater than 1 g/L titer. In the study, four reactors were run simultaneously with four individually controlled systems. Thus, providing validation of the process with all 4 experiments only consuming less than 0.5 L of media in total (Figure 1).
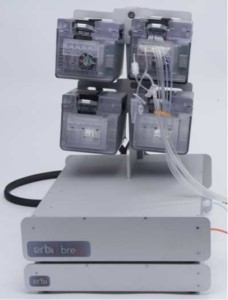
Components of the Breez
Dr. Lee then explained the components of the Breez microbioreactor platform that make it so efficient and how they are able to achieve bench scale stirred tank performance in 2 mL scale working volume.
At the heart of the system are the PODs, which serve as a fully automated controller pod for the microbioreactors. Each POD operates independently and provides mixing and closed loop control for pH, DO, temperature, CO2 and cell density. The pods can operate individually or in parallel.
There is a basestation hub that can hold up to 4 PODs and a CO2 sensor box, which supplies regulated gas pressure, electrical power, and communication to each POD in only 12 linear inches.
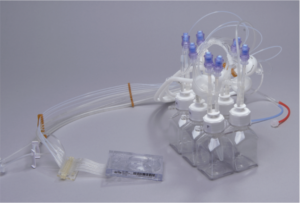
The system employs a sterilized, single-use microbioreactor consumable. The True Perfusion microbioreactor cassette, with fully integrated fluidics, enables quick experimental set up and take down. Since it is a functionally closed system the contamination risk is significantly reduced.
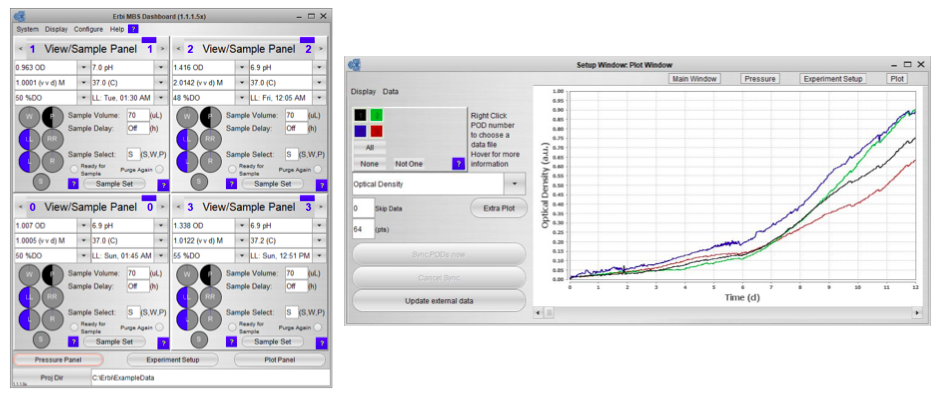
The third component is the intuitive and powerful software. The software makes it easy to set up the experiment, monitor and control the PODs, and the microbioreactor consumable with a standard controller. The interface can execute complex protocols and provides remote software and web access. A data historian with graphing for all key parameters is also included.
Since the bottles, sensors and pumps are integrated into the consumable, the software is also able to manage all aspects of fluid flow, including estimating fluid levels as part of fluid tracking and management (Figure 2).
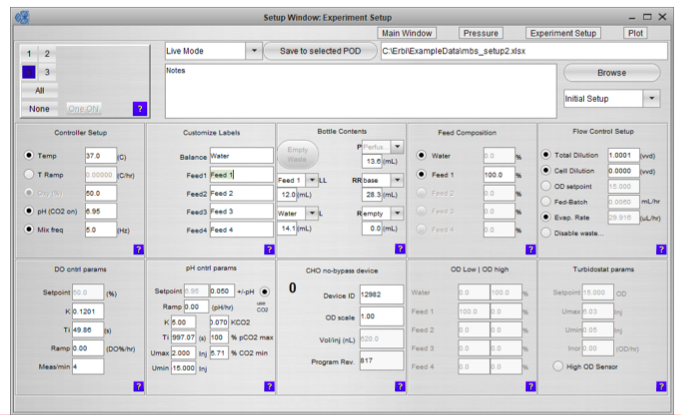
Once the system is inoculated and running you can view the complete process state in a single panel (Figure 3). Graphical bottle volume indicators alert any volumes that are critically low.
Sampling is simplified and completely automated, via a single click. No direct contact between sample cup and reactor provides a completely sterile closed sampling system that can be conducted in a non-sterile space.
Breez Operation
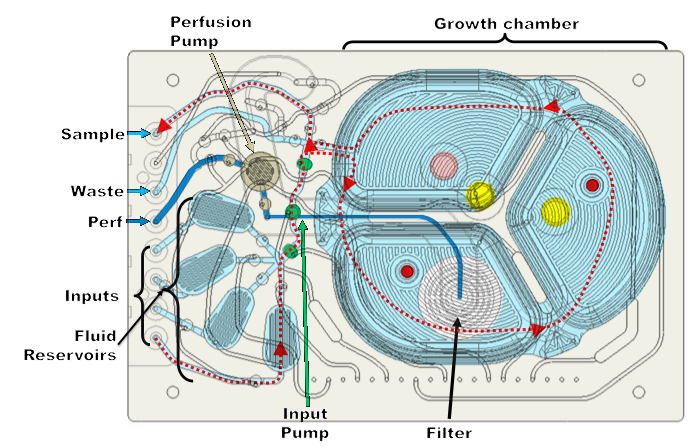
Dr. Lee showed a schematic of the microbioreactor cassette and it is a series of pumps and valves that provide fluid movement with input and output flow (Figure 4). It has an integrated 1.2 μm filter for a clarified harvest, used typically for N-1 perfusion, cell concentration, washing and media exchange, and, of course, continuous operation. There are four liquid and three gas inputs per reactor with kLA up to 40/hr. He then walked through the flow path of the media moving through the reactor.
Fluid Movement
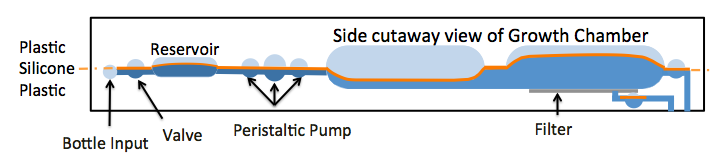
A thin silicone membrane separates upper and lower rigid plastic chambers and drives fluid motion. The flow in the lower chambers is driven by pressure or vacuum in the upper chambers, enabling integration of valves, reservoirs, pumps and mixers. (Figure 5).
Reactor volume is increased by fluid input, and then normalized through an output to maintain a consistent working volume. All flow is counted as part of the settable dilution rate, this includes pH injections and cell bleed. Cell density is On/Off controlled: if optical density is above the setpoint, flow is diverted to the cell bleed in 25 microliter increments (Figure 6).
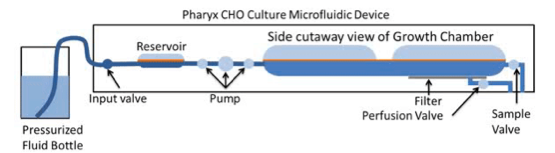
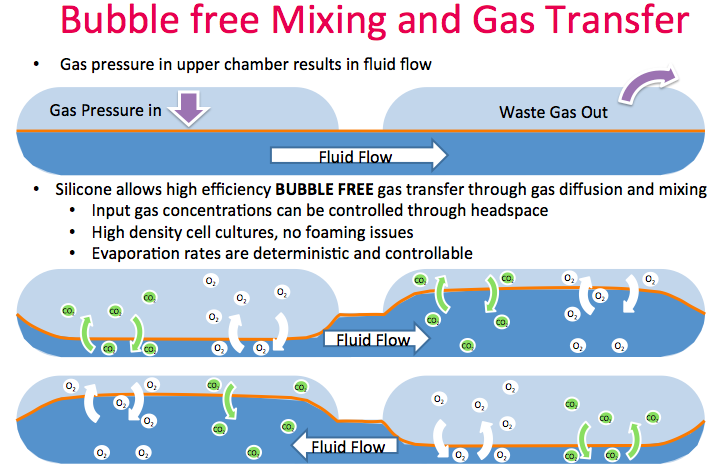
High efficiency bubble free mixing enables efficient oxygen transfer
The flexible silicone membrane enables a unique feature that Dr. Lee called an oxygenating peristaltic mixer. Gas pressure in the upper chamber results in fluid flow with waste gas removed as new gas is introduced. The silicone allows high efficiency, bubble free gas transfer through gas diffusion and mixing (Figure 7). Thus, no need to sparge bubbles or deal with foaming issues. Input gas concentrations can be controlled through the headspace.
Typically, systems this small in volume are prone to evaporation, however since aeration is bubble free and gas transfer only occurs through diffusion, the kLA is very consistent. Evaporation rates are deterministic and controllable with water additions.
Video of media injection and media cycle
Dr. Lee, then showed a video in which it is clear that the mixture becomes homogenous very quickly with mixing times in a few seconds. This video is available on the Erbi Biosystems website (erbi-bio.com).
Integrated sensing
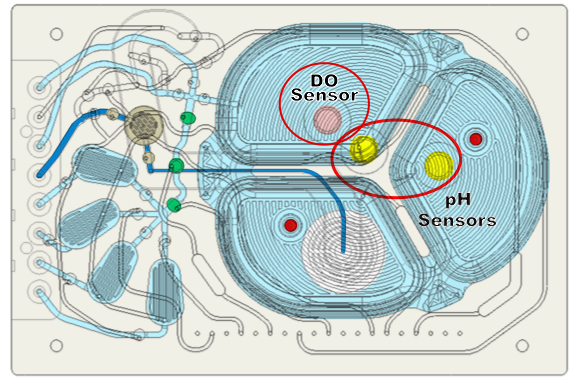
Sensors are incorporated throughout the microbioreactor (Figure 8). Dr. Lee explained that there is a dissolved oxygen sensor that takes measurements every 15 seconds and two pH sensors to ensure long term accuracy. The main pH sensor takes measurements every 2 minutes and the reference pH sensor measures once every 24 hours for drift correction. Erbi has demonstrated pH measurements for 30 days without the need for recalibration.
In addition to the DO and pH sensors, there are two optical density sensors with two different optical path lengths optimized for different densities (Figure 9). They correct drift in optical density measurements in real time using the reference measurements. This significantly reduces the need to take cell samples to measure density.
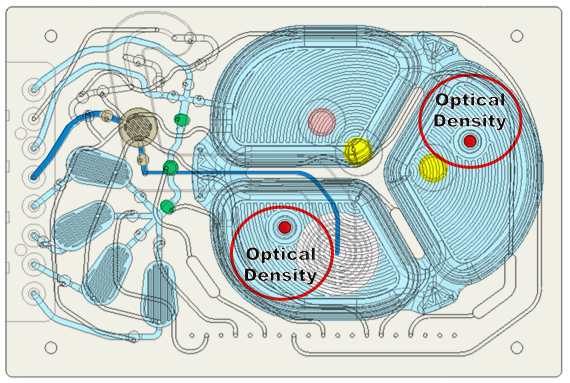
Its novel OD measurement system takes advantage of the inherent movement of the silicone membrane to configure the measurement chamber to either be full of cells or completely empty (Figure 10). In the first configuration, they pressurize the neighboring chamber to move cells into the measurement chamber and take a measurement of cell density. In the second configuration, they pressurize the measurement chamber to remove all liquid and take a reference measurement. The ratio of these measurements is related to cell density and has much better immunity to optical path variations.

Performance
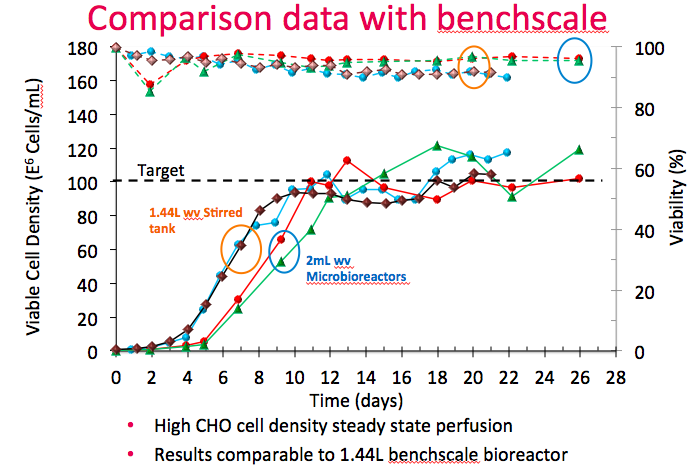
With optical density control, Dr. Lee described how they can achieve steady state perfusion with less work. In figure 11, the Breez is compared with 2 L bioreactors running the same cell line and process. Stirred tank bioreactor performance is shown in brown and blue and the Breez is shown in red and green.
He explained that both bioreactors reached the same steady state with cell density of 100M cells/mL. The main difference was that the stirred tank performed manual cell bleeds based on daily online sampling. The Breez was closely controlled using optical cell density, which required less frequent sampling. Cell viability was also higher in the Breez. (The delayed exponential growth in the Breez was due an error in setting headspace CO2 too low early in the culture.)
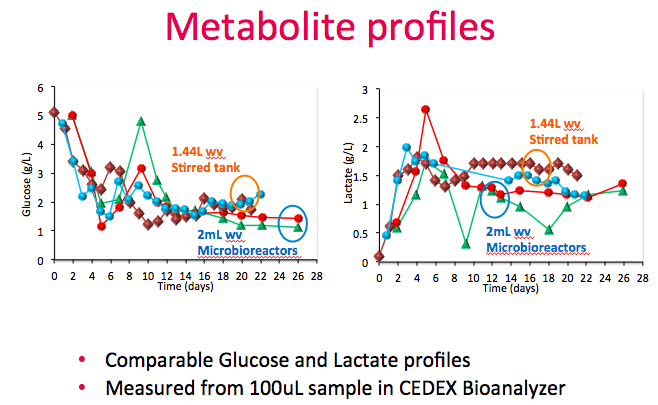
Dr. Lee then showed metabolic profiles for both the stirred tank bioreactors vs. the Breez. In both systems, 100 grams/L of glucose was supplemented as a second feed to maintain culture at less than 2g/L and the glucose and lactate profiles were comparable (Figure 12). Measurements were taken from both the cell sample supernatant and perfusion harvest.
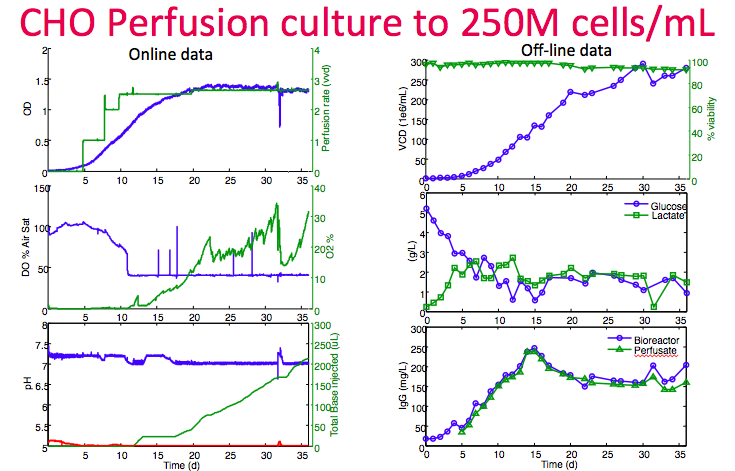
Next, Dr. Lee shared an example of a cell line reaching 250M cells/mL at high viability in the Breez (Figure 13). In the left column, data demonstrates tight control of DO and pH set points. Cell bleed was started on day 32 to show that control is still possible even at this high density. In the bottom right graph, measurements of product titer from the cell sample supernatant and perfusion harvest confirm very minimal filter fouling.
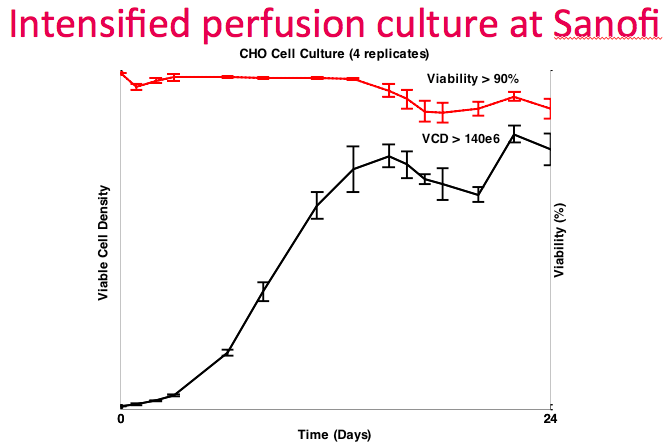
At this point, Dr. Lee revisited the data from the collaboration with Sanofi. They ran four replicate runs, which demonstrated good repeatability. They achieved the target density of greater than 140M cells/mL with greater than 90% viability (Figure 14). They were able to utilize the OD sensor as a proxy for culture growth and did not have to verify with online measurements frequently, thus reducing the amount of sampling required.
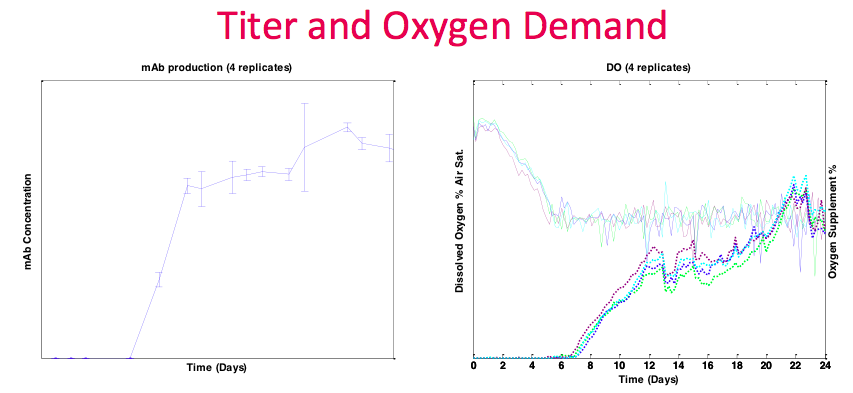
In contrast to the previous example, the Sanofi process ran at higher density and had more demanding oxygen requirements. The Breez was able to handle this oxygen demand with a significant amount of headroom, and still available to accommodate more. Since gas transfer is bubble free, and kLa’s in the Breez system are consistent over time, oxygen supplementation is directly related to oxygen demand and cell activity. In other systems, kLa’s can change during a run due to many factors, such as agitation and bubbles, making correlations between controller outputs and cell activity more difficult to interpret. In the graph, there are also many data points for titer (Figure 15). This is due to the perfusion harvest volume being sufficient to accommodate daily sampling even in 2 mL working volume.
Summary
Dr. Lee reiterated that the Breez can save set up and operation time with single-use, pre-filled sterilized microbioreactor cassettes and an integrated fluid delivery system with automated setup and calibration. No assembly, sterilization or tear down time needed.
Since Breez bioreactors can operate independently or in parallel, they enable individualized automation with greatly higher throughput capacity than bench top reactors. They have an integrated filter to autonomously perform sophisticated control strategies, N-1 perfusion, media exchanges and cell concentration.
Lastly they are able to demonstrate high performance, by achieving and maintaining high cell densities (>200M CHO cells/ml) over 30 days with integrated pH, DO, temperature and cell density control. Thus, the Breez system provides a robust scale down model for large-scale processes with 1000-fold reduction in media and reagent costs, and with a higher experimental throughput per person.