
The Next Evolution in Stem Cell Culture™: Cell-Derived Extracellular Matrices
The interaction between cells and the extracellular matrices they are cultured on is extensive. In standard cell culture, cells are pulled from tissue, which provides a native extracellular matrix and are put onto tissue culture treated plastic. While most cells will ultimately adhere and grow on tissue culture treated plastic, their gene expression and proliferation is often different from in vivo.
In this mini-webinar and accompanying article, Sy Griffey, Ph.D. Chief Operating Officer, StemBioSys, discusses cell derived extracellular matrices as the next evolution in stem cell culture. This cell derived matrix provides cells with a substrate that closely mimics the biochemical and structural microenvironment found in vivo.
Dr. Griffey began the webinar by discussing the ingredients for cell culture, tissue regeneration, and tissue engineering. In this system, tissue culture treated plastic is often used as the cell attachment surface. Dr. Griffey explains that cell and matrix interaction is extensive and artificial surfaces do not address the needs of the cells and thus cells are forced to lay down their own matrix, which causes them to behave differently than they would in vivo.
Sy goes on to explain that in the 1960’s the industry moved to polystyrene based cultureware and since that time, there has been hasn’t been much evolution. To address the need for an improved extracellular matrix that was more native-like, StemBioSys developed their CELLvo™ Matrix, which is a naturally produced extracellular matrix (ECM) of proteins synthesized in vitro by bone marrow stromal cells.
To view the webinar, please click play below:
CELLvo Matrices Production
Dr. Griffey then explained that CELLvo Matrices are made using a bank of commercially available bone marrow stromal cells from healthy young adult donors. These cells are added to cultureware and are cultured with a proprietary cell culture media. Cells begin to divide and cover the cell culture dish or other substrate. Cells are then stimulated to create an extracellular matrix with matrix proteins deposited in a defined and ordered 3D structure. Thus creating a complex multi-protein composite that mimics the natural bone marrow niche for stem cells. After production, bone marrow cells are gently removed so as to not impact the matrix architecture or biochemistry. The matrix is thoroughly washed, solutions removed, and vessels dried leaving behind a native ECM. Analysis of the finished product confirms the complex nature of the niche with over 100 different proteins laid down by the cells. The final product is cell free with only the ECM attached to the surface of the culture vessel. CELLvo Matrices are available in two product types, CELLvo Matrix and CELLvo XF-Matrix and can be used to culture a variety of adherent mammalian cells.
CELLvo Matrices Performance
Sy stated that one of the questions that he gets asked most frequently is how consistency is ensured, if the source cells are collected from different donors. He explained that while there are over 100 different proteins present in the matrix, the top 20 proteins quantitatively are always the same from donor to donor. In fact, Sy presented data showing that mesenchymal stem cells (MSCs) cultured on CELLvo Matrices demonstrated shorter doubling times and exhibited less variation in growth potential from donor to donor when compared to standard tissue culture plates. (Figure 1)
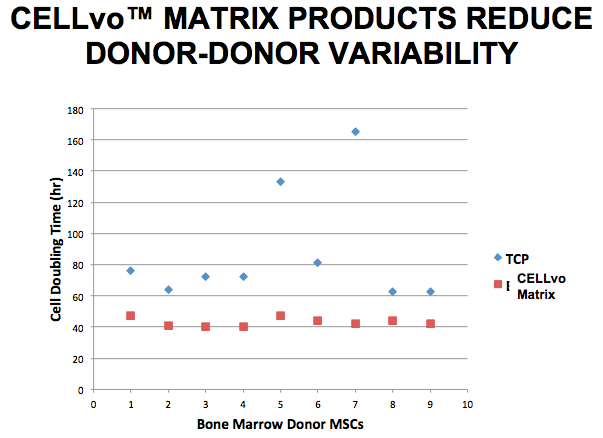
Dr. Griffey hypothesizes that this reduction in variability and increased proliferation is a result of the more natural matrix. Cells do not have to spend time and resources laying down a self-generated matrix and can more rapidly adapt to the culture environment.
Dr. Griffey went on to state that their data is not limited to MSCs derived from bone marrow. He presented data on performance of MSCs derived from cord blood, cord tissue, adipose tissue and amniotic fluid. Additionally, CELLvo Matrices have also been used to successfully isolate and expand other cell types including chondrocytes, endothelial cells, cardiomyocytes, human islet cells, cancer, germ and renal cells, macrophages and human islet cells, and many others. (Figure 2).
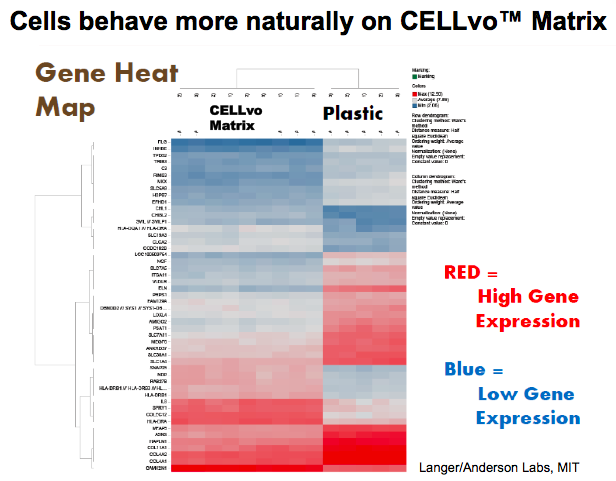
Dr. Griffey went on to state that their data is not limited to MSCs derived from bone marrow. He went presented data on performance of MSCs derived from cord blood, cord tissue, adipose tissue and amniotic fluid. Additionally, CELLvo Matrices have also been used to successfully isolate and expand other cell types including chondrocytes, endothelial cells, cardiomyocytes, human islet cells, cancer cells and germ cells.
Advantages of CELLvo Matrices
In closing Sy summarized the primary advantages of CELLvo Matrices:
- Provides a natural substrate for cell isolation and growth
- Allows cells to be more responsive to experimental variables
- Other substrates only contain one or a few ECM proteins
- CELLvo™ Matrix is ready to use and is the only substrate built by cells for cells
Furthermore, StemBioSys has also found that the cells cultured on CELLvo Matrix are unique and exhibit a more natural phenotype than cells isolated and grown on tissue culture treated plastic. StemBioSys now offers these cells as part of their product offering with the following cells available for purchase:
- Cord blood derived EPCs
- Chondrocytes
- Bone marrow derived MSCs
- Wharton’s Jelly derived MSCs
- Adipose derived MSCs
In addition to CELLvo Matrices and CELLvo Cells, StemBioSys is also developing CELLvo Cell Culture Media, specially designed to be used with CELLvo Cells.
For more information, please see http://www.stembiosys.com/cellvo-matrices or email infor@stembiosys.com
About the Presenter:

Sy Griffey, Ph.D. Chief Operating Officer, StemBioSys
Prior to joining StemBioSys Dr. Griffey was with Kinetic Concepts (KCI) in San Antonio for over 8 years, most recently serving as Sr Director in KCI’s Center for Advanced Research and Technology. Previous positions at KCI included Director, New Product Concept Development and Principal Scientist, Biosurgery Research.
Prior to KCI Dr Griffey spent 15 years with LifeCell Corporation serving in various roles of increasing responsibility from basic research to new product concept development to product and process development. Dr Griffey’s primary area of emphasis over 25+ years in industry R&D has been in assessing new ideas and technologies and evaluating the feasibility of turning these ideas into medical devices and products. His research and product development background includes programs and projects involving medical devices, stem cells, tissue regeneration and biologic transplant materials for orthopedic, reconstructive, sports medicine and wound healing applications. Revenues generated from products that were directed by Dr Griffey at various points in their development cycle are in the $100’sM of revenue per year.
Dr Griffey received his bachelor’s and master’s degrees at Southwest Texas State University and received his doctorate from Baylor College of Medicine in Houston with a joint appointment in Cell Biology and Biotechnology.