
Examining Bioprocessing Scale-Up Challenges and Technology Solutions
The number of novel cell and gene therapeutics gaining regulatory approval is on the rise to treat a variety of human diseases. Yet, these complex biologics-based therapies present challenges for drug companies as to the best approach to increase the manufacturing volumes in a cost-effective for mainstream use. Scale up and scale-out are the two main strategies utilized in the industry and the best choice can depend on several factors including the type of therapeutic, unit operation, and available technologies.
In partnership with BioPharm International, Eppendorf has released e-book focused on “Addressing Bioprocessing Scale-Up Challenges”, a compilation of four articles that examines industry perspectives and technology solutions to overcome unique scaling challenges associated with the production of biologics, including cell and gene therapies.
New Therapies Present Scaling Challenges
In the first article, Feliza Mirasol from BioPharm International discusses how emerging cell and gene therapies present unique scaling challenges compared to traditional biologics such as monoclonal antibodies (mAb). Because the cells are themselves the therapeutic agent, they must maintain their integrity and functionality upon scale-up, which means different strategies are required to increase their production. As the cell density in the biomanufacturing environment increases, so too does the potential to introduce inhomogeneity into the system, which can impact the quality and effectiveness of the cell therapy.
Cell therapies can be broadly categorized as autologous (patient-derived) or allogeneic (donor-derived). Because autologous therapies utilize a patient’s own cells in the manufacturing process, it is not easily scalable because each treatment is patient specific. Commercial autologous cell therapy production requires a decentralized scaled-out manufacturing loop—from patient to manufacturing facility and back to patient. Lowering costs relies on advanced engineering and automated manufacturing solutions that reduce the complexity and labor-intensive (and costly) steps in the current workflow. Bioprocessing equipment and materials suppliers are actively developing suitable tools and processes to meet the manufacturing challenges of these personalized treatments.
In contrast, allogenic cell therapies use a single batch of healthy donor cells to produce multiple doses of the therapeutic for many patients, operating in a manner more similarly to mAbs and are more suitable for traditional scale-up route to offer economies of scale. Additionally, a wealth of institutional knowledge and enabling technologies used for scaling up mAb production can be applied to support comparable approaches for allogeneic cell therapies, provided that a product quality profile for the allogenic cell therapy has been established and is well understood.
Likewise, gene therapy manufacturing has similarities to mAb manufacturing that allow for some steps in its process to be scaled up, particularly in the downstream purification unit operations. However, the transient transfection step is highly complex and remains a major challenge to scale-up because the processes are still being refined. As well, inherent limitations on how much DNA each cell can take up restricts both the DNA concentrations and cell densities that can be achieved impacts the potential scaling approaches. For instance, the existing bioprocess for Spark Therapeutics’ gene therapy product Luxturna, developed to treat Leber congenital amaurosis, uses adherent cells grown in a roller bottle system and a scale-out strategy is used to increase production volume. Multiple parallel units are used at a certain scale and material is combined at the final step, after the product has been purified to generate large batches of the gene therapy. Spark is looking toward implement more intensified process solutions with better efficiencies for future production requirements as their knowledge of the process continues to build. In the case of the cell culture process, it is moving toward a suspension bioreactor cell culture to replace legacy attachment-dependent cell culture to improve productivity and gain flexibility in scale-up and scale-out capabilities.
As industry knowledge continues to increase, developers will be able to better apply engineering principles to overcome the biochemistry, the virology, and the molecular biology associated with these complex biologics.
Scale-Up of Escherichia coli Fermentation from Small Scale to Pilot Scale Using Eppendorf Fermentation Systems
The second article focuses on fermentation processes, which are critical to the success of industrial production of biologics in the biopharmaceutical market. For gene therapies, E. coli fermentation is used to produce the plasmid DNA needed for the transient transfection of mammalian cell lines to generate viral vectors. It is necessary to increase production volumes to keep pace with the increasing demand for viral vectors and having vertically scalable production platforms can help expedite scaling procedures.
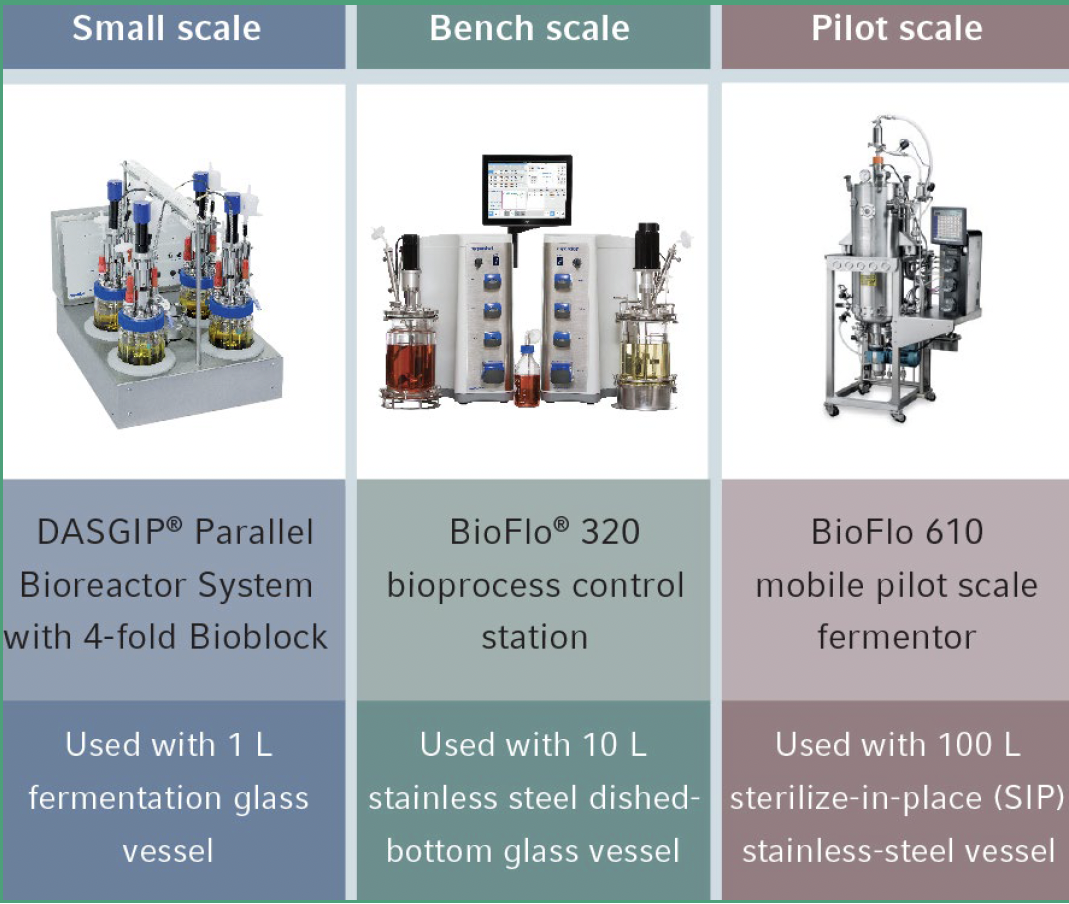
Eppendorf scientists use E. coli fermentation to demonstrate the scale-up capabilities of Eppendorf fermentation systems from small- to bench- to pilot-scale and highlight critical scalability-related engineering parameters. Optimizing processes at production scale is cost-prohibitive so performing these studies in scaled-down bioreactors and fermenters is preferred. Once a process is optimized at smaller scale, the parameters can be transferred to pilot scale following established scale-up strategies. Eppendorf fermentation systems are designed following the same vessel and impeller geometrical principles, offering vertical scalability from small- to large-scale stirred-tank fermentation to speed process development.
Critical engineering parameters such as oxygen transfer rate (OTR), power number (Np), and impeller power consumption per volume (P/V) were evaluated, before the data was used to design a scale-up strategy following the constant power (P/V) principles on the Eppendorf fermentation systems.
Oxygen Transfer Rate (OTR): OTR is the rate at which oxygen is transferred from air to the liquid in a vessel, and because oxygen is often the limiting factor during aerobic fermentation, it is important to select equipment of different sizes with high OTR capabilities so that the different fermentation scales can match each other in top line performance, and the small-scale success can be replicated in large scale.
Various strategies can be used for fermentation scale-up including constant tip speed; one method is constant power (P/V), which requires the determination of impeller power numbers (Np).
Impeller Power Number (Np): Np is commonly used to calculate impeller power consumption during bioprocess scale-up. P/V can be calculated from Np using the following equation:
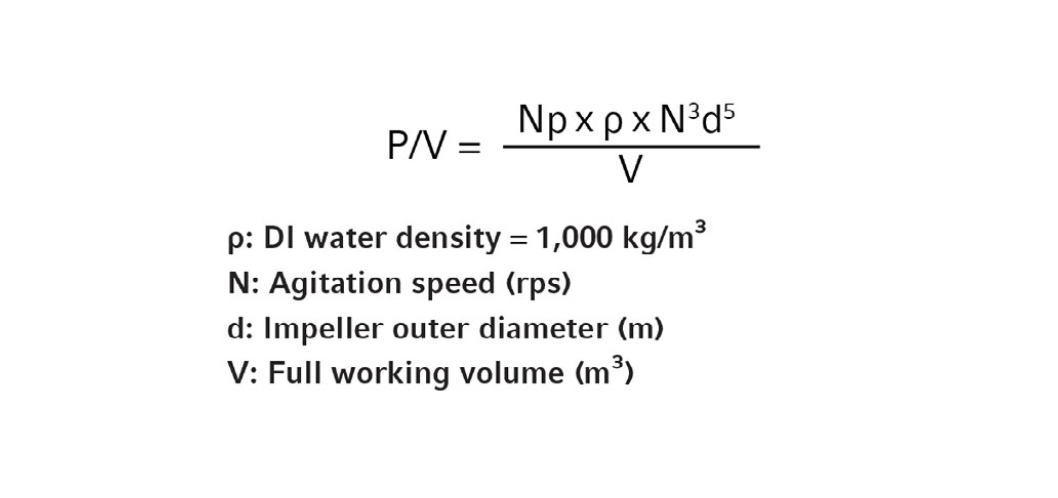
Often, power numbers are determined in the absence of gassing; however, since gassing greatly reduces impeller torque, it is worthwhile to obtain Np under high gassing condition (typical of high-density fermentation) in addition to “no gassing” conditions. For instance, the impeller Np for the Eppendorf fermentation vessels were ~10 without gassing and ~5 with 1.5 VVM of air sparging.
In the studies, Np numbers were calculated at different tip speeds up to 3.8 m/s to determine the constant P/V value for scale-up. The maximum P/V achievable by all three scales was ~2.42 kW/m3 based on Np values obtained under 1.5 VVM and was selected as the constant P/V value for the scale-up design. Maintaining constant P/V at 2.42 kW/m3 between different fermentation vessel sizes from 1 L (controlled with a DASGIP Parallel Bioreactor System) to 10 L (controlled with a BioFlo 320 bioprocess control system) to 100 L (controlled with a BioFlo 610 bioprocess control system) produced nearly identical E. coli growth curves, with OD600 at or near 140 within 9 hours of inoculation providing solid proof for the scalability of Eppendorf fermentation systems (Figure 2).
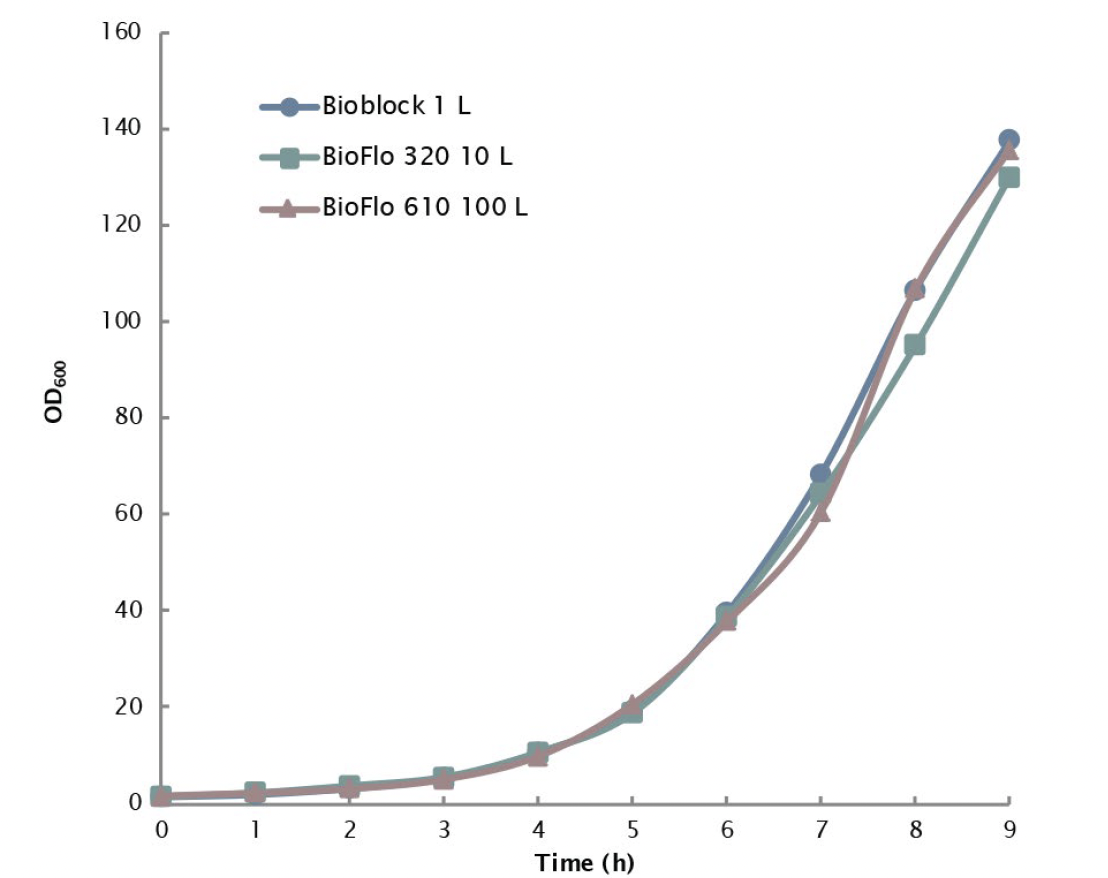
All three systems can deliver high OTR values, providing excellent capability for high density aerobic fermentation in a scalable manner.
Bioprocessing Scale-Up Challenges and Eppendorf’s Newest Solution: Thought Leader Q&A
In the third article, BioPharm International sat down with Ma Sha, PhD, Head of Bioprocess Applications at Eppendorf, to discuss current trends in bioprocess scale-up, such as the shift from constant kLa to power over volume (P/V) as a scaling strategy and enabling technology solutions such as Eppendorf’s new BioFlo 720 bioprocess control system. The key take-aways from the interview include:
1) The gold standard scale-up strategy has switched from using constant kLa to using constant P/V. For the past five years, Eppendorf has been using the constant P/V method because it is more be efficient and requires fewer measurements than kLa. It requires only a single constant to be measured: the Power Number (Np). The power number is used to calculate P/V, and it drastically reduces the upfront efforts needed to achieve matching yields between scales.
2) Because P/V scale-up is still quite challenging and requires significant expertise, smaller companies may not have the resources to accomplish it effectively. That’s why Eppendorf has brought its scale-up expertise into the new BioFlo 720 bioprocess control system using a software innovation called Scale-Up Assist. The software internally calculates constant power, ranging from Eppendorf’s BioBLU Single-Use Bioreactors on the small and bench scale all the way up to the 2,000 L pilot-scale Single-Use Bioreactors. A customer can enter small run parameters, and the BioFlo 720 will predict the large-run parameters automatically to obtain matching biomass and protein production yield.
3) The BioFlo 720 bioprocess control system was designed to maximize usability.
- It is compatible across three different types of dissolved oxygen (polarographic, digital polarographic and digital optical DO) sensors with an Auto Calibrate feature that automatically executes the calibration.
- It is the first Eppendorf controller to use ThermoFisher Scientific’s pilot-scale cell culture Single-Use Bioreactor (SUB) with an Auto Inflate function that enables walk-away capabilities for inflating very thin, plastic single-use bags.
From Shaker to Pilot/Production Bioreactor: How Scale Up Assist Using the BioFlo® 720 Bioreactor Control System Can Help Your Antibody Production Workflow
In the final article of the e-book, Eppendorf scientists use a standard CHO production process to demonstrate how the new BioFlo 720 bioreactor control system can be used to streamline the scale-up of mAb production while maintaining cell culture growth and batch yields at large working volumes. All experiments used a proprietary suspension CHO cell line producing a human monoclonal antibody (hmAb) from TPG Biologics, Inc., cultivated in Dynamis™ AGT™ Medium (Thermo Fisher Scientific®).
The built-in software algorithm on the Scale Up Assist feature automatically calculates all critical parameters needed, including gas flow rates and agitation set points to use for different vessel sizes to match CHO growth curves and mAb production yields. The team tested the Scale Up Assist feature to successfully scale CHO batch cultures from 3 L to 10 L BioBLU vessels to 50 L Single Use Bioreactor (SUB) bag.
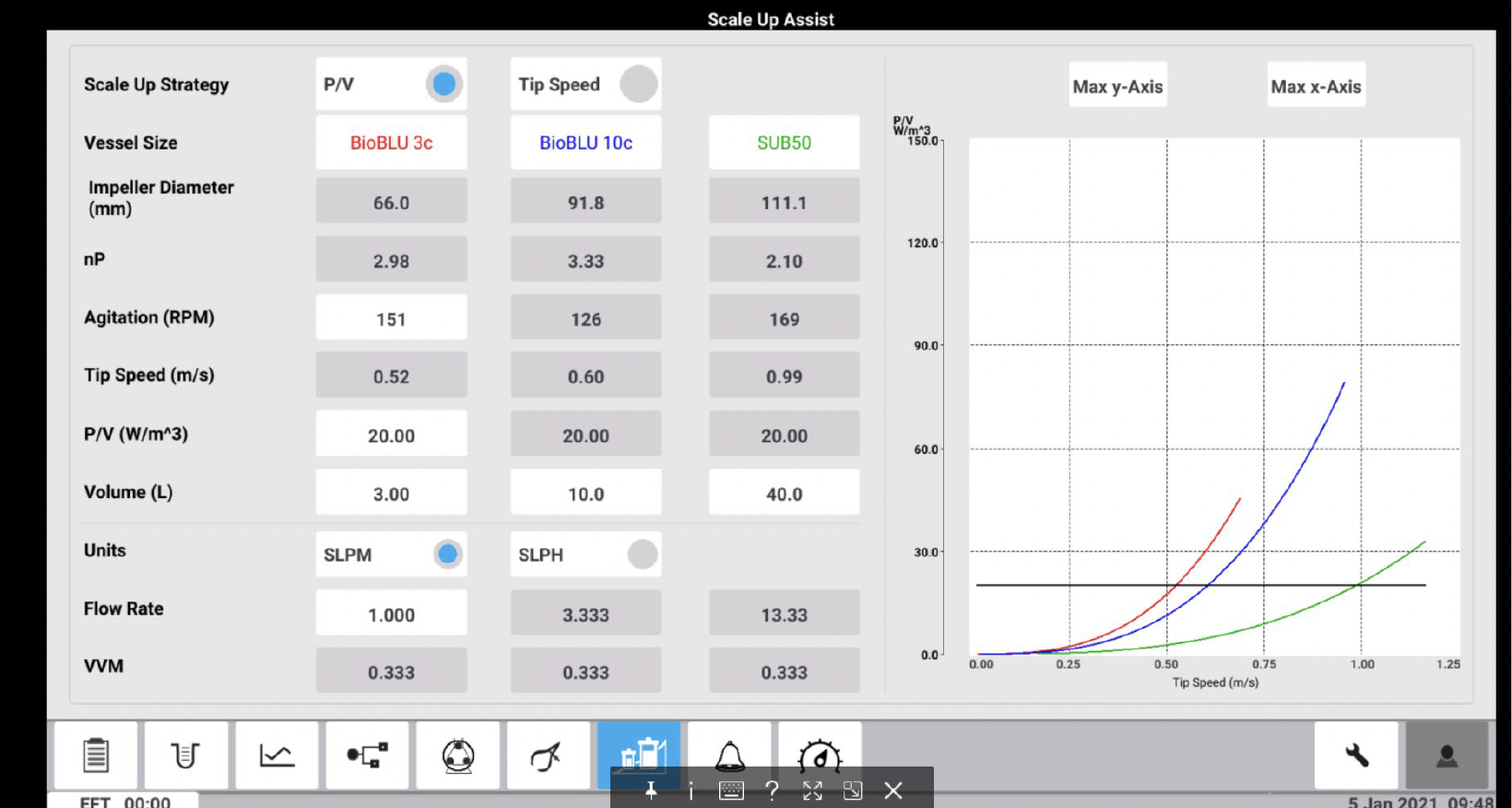
The bioreactors were sampled twice daily to monitor metabolic profiles with values collected for parameters such as cell density, viability, glucose, ammonia (NH3), lactate, and hmAb concentration. Using the parameters calculated by the BioFlo 720 Scale Up Assist feature allowed the team to match the growth profiles and antibody yields across all platforms in multiple batch runs (Figure 4A and B).
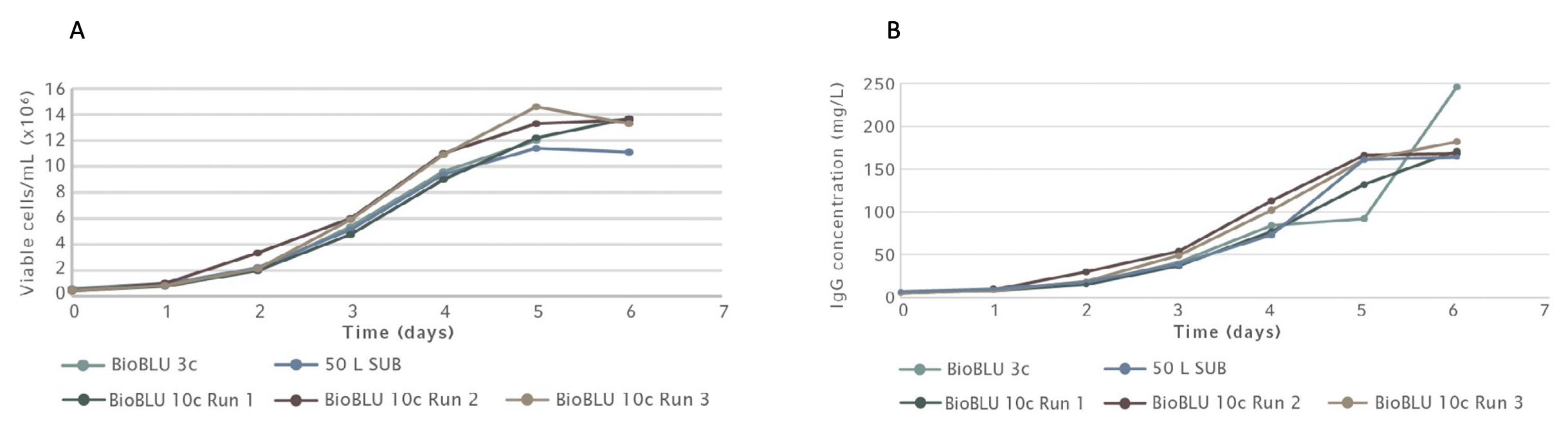
The BioFlo 720 Scale Up Assist application is convenient and straight forward to use, allowing operators to easily tailor their processes. Users select their vessels from the drop-down menus and the Scale Up Assist then automatically calculates the critical parameters, such as agitation and maximum gassing, to successfully scale their culture from one vessel to another. These results of the study presented demonstrate that the new Scale Up Assist feature can effectively maintain cell yields across vessel sizes and bioreactor helping to save production costs, optimize ideal conditions for cultures, and shorten time to market.
Overall, the e-book does an excellent job of highlighting some of the key challenges faced by drug developers as they seek to bring cell and gene therapies to market quickly to meet the demand. Overcoming bioprocess scale-up challenges will go a long way to improving the speed to market in a cost-effective way that benefits the industry and patients too.