ExpiSf™ Baculovirus Expression System Demonstrates High Yield Protein and Viral Vector Production from Micro to Large Scale
Vaccines and gene therapies have a critical role to play in the future of medicine. As seen with the current Covid-19 pandemic, rapid development and manufacture of vaccines and viral vectors can have a tremendous impact on our ability to address and treat emerging healthcare needs. Scalable and efficient protein and viral vector production are essential to the development of vaccines and gene therapies. However, this often presents challenges, as expression systems don’t provide the versatility required to meet multiple formats or production requirements.
Expression Systems
Effectual protein and viral vector production requires selection of a versatile and highly productive expression system. The insect based Baculovirus Expression Vector System (BEVS) has shown its utility in large-scale production of both proteins and viral vectors. A recent poster, Scalable protein and AAV production in the ExpiSf™ Expression System, demonstrates the use of the Gibco™ ExpiSf™ Expression System for high-yield production of proteins and viral particles with consistent performance using a fast, streamlined workflow. The system demonstrates scalability for production in stirred tank, rocking motion, and microbioreactors. Optimal bioprocessing parameters and protocols to enable high quality process development for production of targets of interest are described.
Poster Highlights
Scalable Protein Expression with High Yield
In the study, protein expression was optimized for scale down (ambr 15) and scale up (rocking motion and stirred tank bioreactor) production in the ExpiSf Expression system. Please see poster for specific protocols used, including optimized parameters for each production format. Authors demonstrate that optimization of bioprocess parameters resulted in similar protein titers in scale down and scale up formats compared to shake flask controls. In addition to scalability, authors demonstrated high protein yield in both rocking motion bioreactors and stirred tank bioreactors.
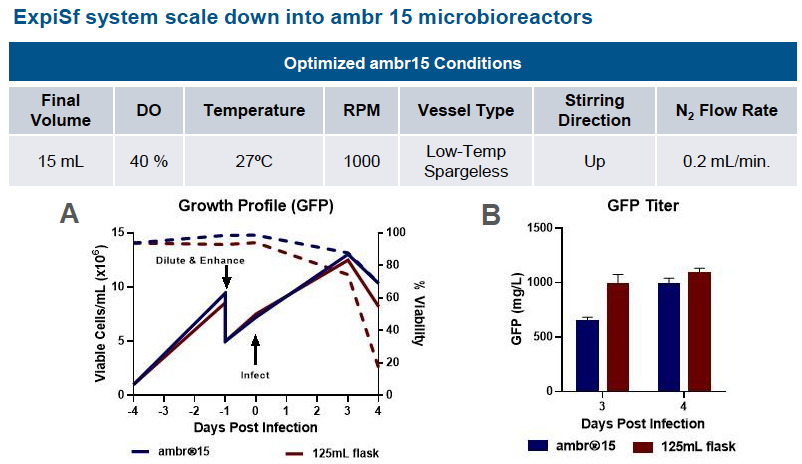
Scale Down Protein Production – Microbioreactors
In the poster, data supports the ExpiSf9 culture system’s scalability. In runs using the ExpiSf Expression system in ambr 15 microbioreactors with shake flasks as the control, viable cells and percent viability were similar. GFP titers in ExpiSf9 cultures for both ambr 15 microbioreactors and shake flasks were comparable (Figure 1).
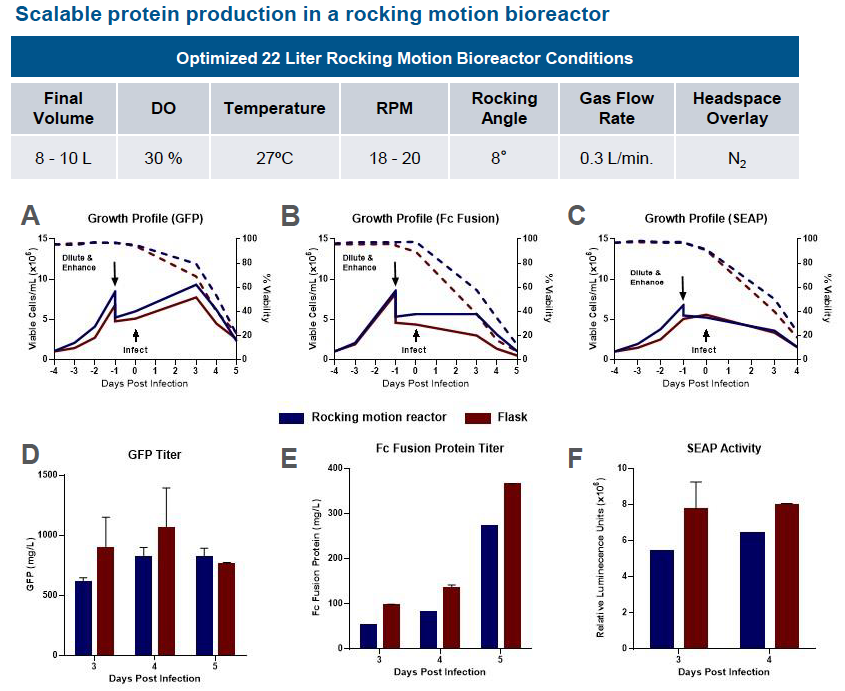
Scale Up Protein Expression – Rocking Motion Bioreactors
The ExpiSf9 system seamlessly scaled up to 22 L rocking motion bioreactors. To evaluate viable cells, percent viability and protein expression, the authors evaluated protein expression runs of GFP, Fc Fusion protein, and Secreted Alkaline Phosphatase (SEAP). They found similar growth profiles for all when comparing shake flasks to the rocking motion bioreactors. At least 80% protein yield was achieved in rocking motion bioreactors when compared with shake flasks (Figure 2).
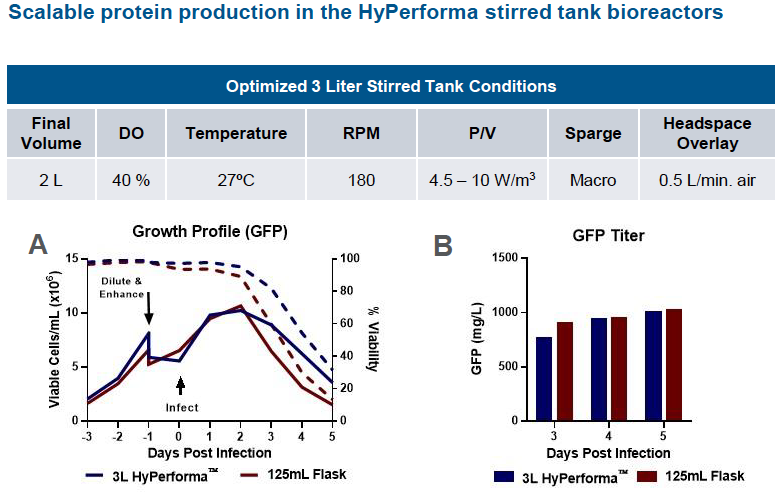
Scale Up Protein Production – Stirred Tank Bioreactors
The authors next looked at scaling the ExpiSf9 system to 3 L HyPerforma stirred tank bioreactors with 125 mL shake flasks as the control. They demonstrated comparable growth kinetics and GFP titers for the 3L bioreactor scale compared to 125 mL shake flask control. (Figure 3).
Scalable and High Yield AAV Production
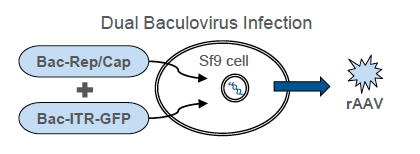
In the poster, the authors also provide data on production and purification of infectious rAAV particles. A dual-infection method was adopted to evaluate the production of two different recombinant adeno-associated virus (rAAV) serotypes, AAV2 and AAV6, in the ExpiSf system. For this purpose, ExpiSf9 cells were infected with two baculoviruses: RepCapX, where X denotes serotype 2 or serotype 6, and inverted terminal repeat (ITR)–green fluorescent protein (GFP). The RepCap baculovirus supplies the necessary viral genes for genome replication, genome packaging, and capsid assembly. The ITR–GFP baculovirus contains the gene of interest (encoding GFP) flanked by AAV ITR sequences.
Scale Up AAV Production – Stirred Tank Bioreactor
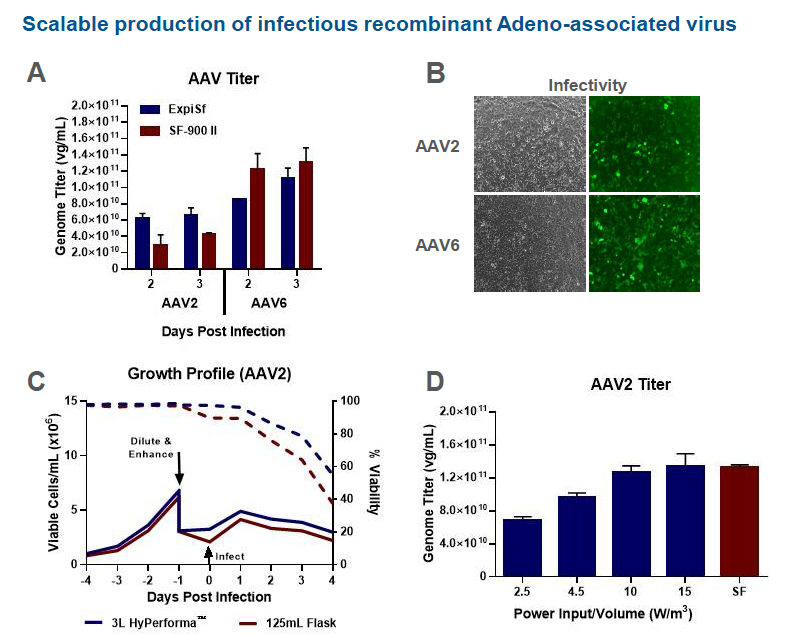
In the poster, the authors looked at production of rAAV2 and rAAV6 using the ExpiSf9 system in 3 L HyPerforma stirred tank bioreactors vs. 125 mL shake flasks. Comparable growth kinetics were seen in AAV2 production for 125 mL shake flasks and 3L HyPerforma stirred tank bioreactors. AAV2 genome titers of crude lysates were measured at 3 days post infection. Production runs in the 3 L HyPerforma stirred tank bioreactor with Power Input per Volume values of 10 and 15 W/m3 were equivalent to the 125 mL shake flask control. Lastly, light and fluorescent images of HT1080 cells inoculated with the rAAV2 and rAAV6 ExpiSf crude lysates confirmed that rAAV from ExpiSf9 crude lysates are infectious (Figure 4).
rAAV Purification
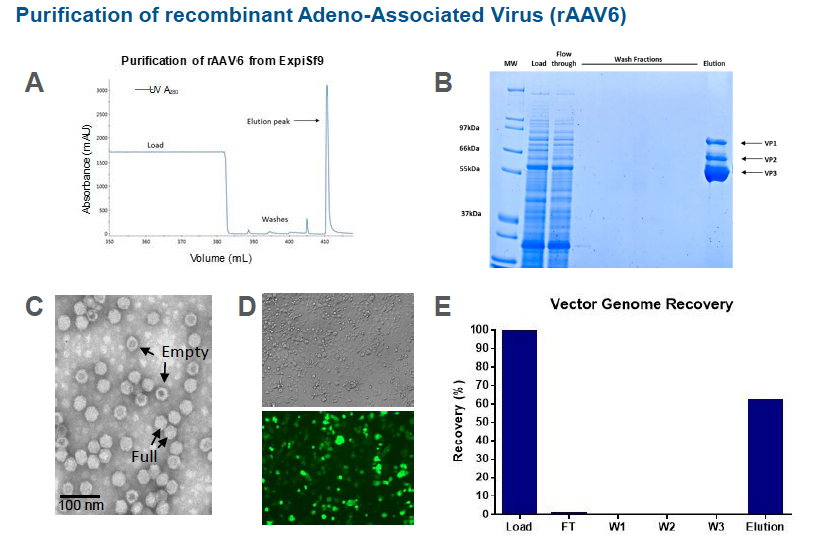
Purification of rAAV6 from the ExpiSf9 production run was performed and an ÄKTA chromatogram showed an elution peak of rAAV6 purified on POROS Capture Select AAVX affinity resin. Fractions from the AAV6 purification run on a Coomassie stained gel show the capsid proteins VP1, VP2, and VP3 are present. A transmission electron microscope image of the purified rAAV6 show empty and full particles. Vector genome recovery was also good at 60%.
Summary
The authors demonstrate the adaptability of the ExpiSf Expression System to a number of formats and scales for the production of recombinant proteins and viral vectors. Optimization of bioprocess parameters resulted in similar protein titers in scale down (microbioreactor) and scale up (rocking motion and stir tank bioreactor) formats compared to shake flask controls. Finally, the production and purification of infectious rAAV particles emphasizes the versatility of the ExpiSf Expression System.
For more information on the ExpiSf Expression System, please see: www.thermofisher.com/expisf
This post is sponsored by Thermo Fisher Scientific.