Glycosylation Overview and How to Control Glycosylation using In Vitro Glycoengineering
Introduction
Glycosylation is the most common type of post translational protein modification. It is also an important factor in manufacturing therapeutic proteins, depending on the mode of action, because it can impact protein stability, biological activity, and the pharmacokinetics of the final product. Due to the impact that glycosylation can have on the therapeutic, it is frequently identified as a critical quality attribute. Thus, it is important to control glycosylation during manufacturing to ensure consistent glycosylation for the final product.
In researching this issue, I found a very informative webinar on the subject. The webinar, “In Vitro Glycoengineering – Taking Control of Glycosylation,” provided both an introduction of glycosylation and information about controlling glycosylation using in vitro glycoengineering.
Glycosylation – Impact on Therapeutic Proteins
The webinar began with Roland Dorn, International Product Manager, Roche Custom Biotech, Roche Diagnostics, providing a nice background on what glycosylation is. I found this to be a very helpful overview. He began by describing the two types of glycosylation:
- N-glycosylation: sugars attached to an asparagine residue
- O-glycosylation: sugars attached to a threonine or serine residue
For the purposes of this talk, he focused on N-glycosylation.
Mr. Dorn went on to describe the role of glycosylation in stability and function of glycoproteins. Key areas of impact include:
- Protein – folding and stability
- Biologic activity – for example antibody dependent cellular cytotoxicity (ADCC)
- Clearance – pharmacokinetics
Mr. Dorn then provided a nice example of the effect of glycosylation on clearance of erythropoietin (EPO) by citing a published paper that demonstrated EPO with intact glycosylation stayed in rat plasma up to 30 minutes or longer. However, desialylated EPO was cleared from rat plasma within minutes.
Glycosylation in Biomanufacturing
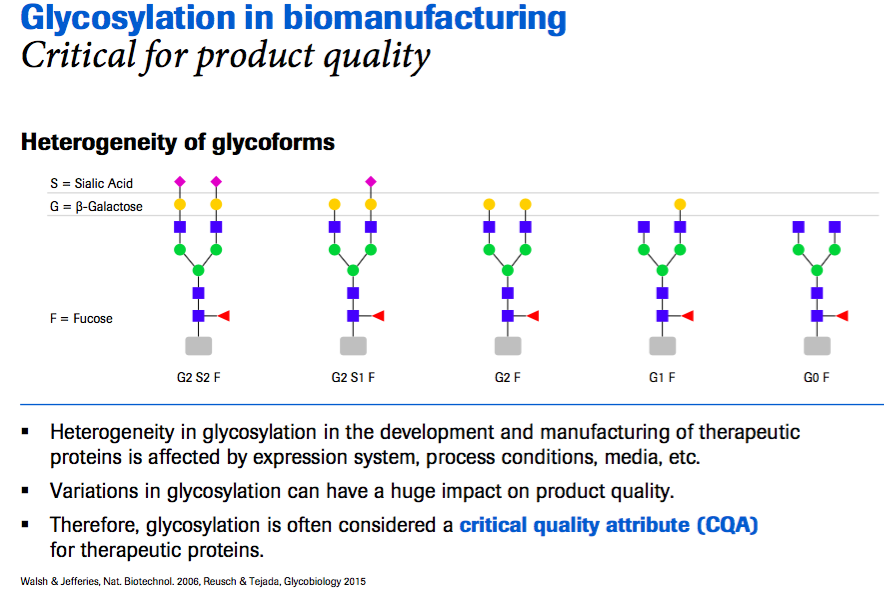
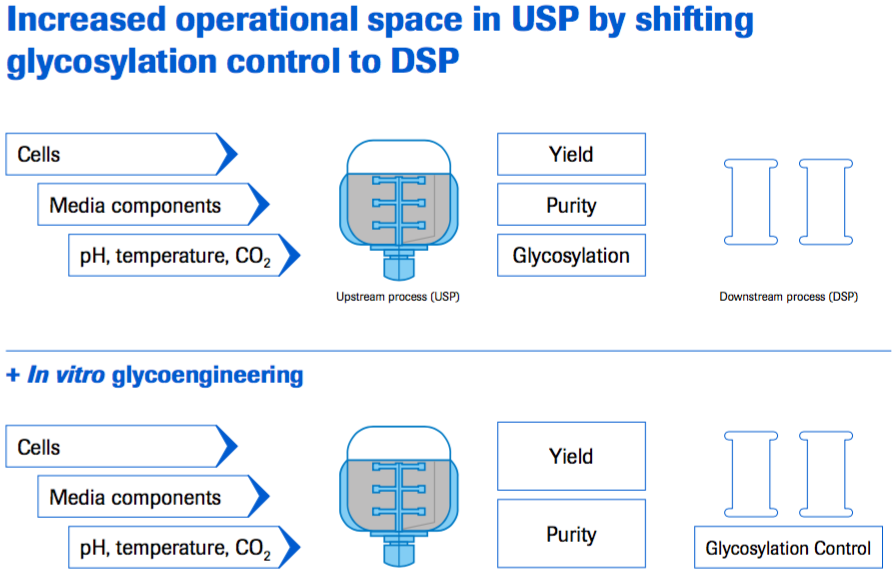
Mr. Dorn then explains how glycosylation impacts expression of a therapeutic protein. He states that “when expressing a therapeutic protein often you will probably have several variants of the glycan called glycoforms”(Figure 1). Heterogeneity occurs in the development and manufacturing of therapeutic proteins and can be affected by expression system, process conditions, media, etc. These variations can have an impact on product quality and so glycosylation is often identified as a critical quality attribute (CQA) in biomanufacturing.
Technologies to Guide Glycosylation
There are ways to guide glycosylation to create a specific pattern and decrease variability. Mr. Dorn described the following:
At the cellular level, you can use genetic engineering to either over express or silence enzymes involved in glycosylation. During fermentation, you can use process engineering e.g. regulate galactose feed to reach higher levels of glycosylation. Both of these methods are routinely used in Biomanufacturing. However, they involve adjusting multiple parameters that can influence other aspects of manufacturing including product yield and other quality attributes. In addition the amount of influence over the glycosylation can be limited depending on the system being used.
Mr. Dorn then described another option, a new technology, in vitro glycoengineering (IVGE). In this technology, instead of engineering the cell or the fermentation process, you engineer the protein of interest at the pre-purified state. Through controlled reactions using glycosyltranserases with known kinetics, in vitro glycoengineering offers greater than 90% degree of influence.
Controlling Glycosylation with In Vitro Glycoengineering (IVGE)
In looking at any new biomanufacturing technology, one of the first things to consider is whether this is appropriate for drug manufacturing.
Level of Glycosylation Control
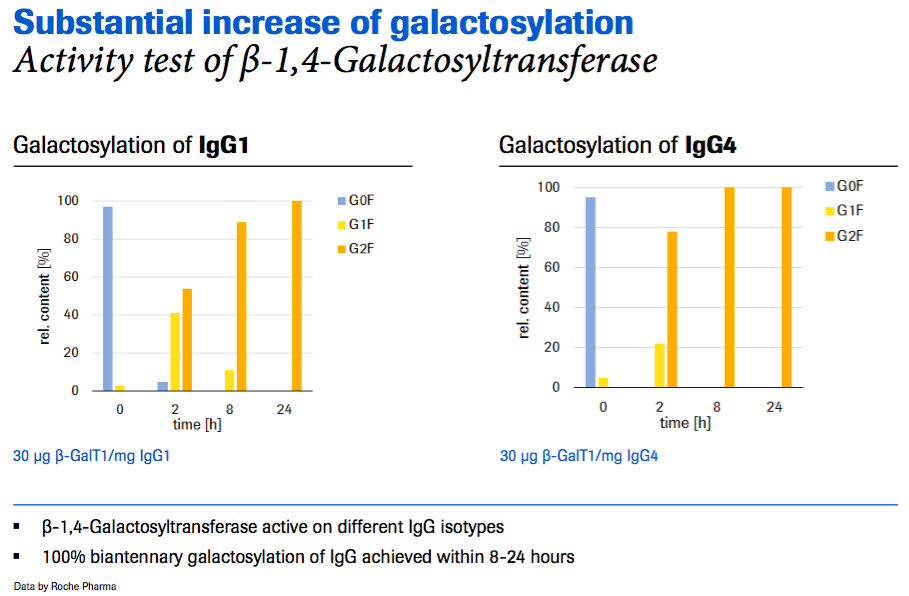
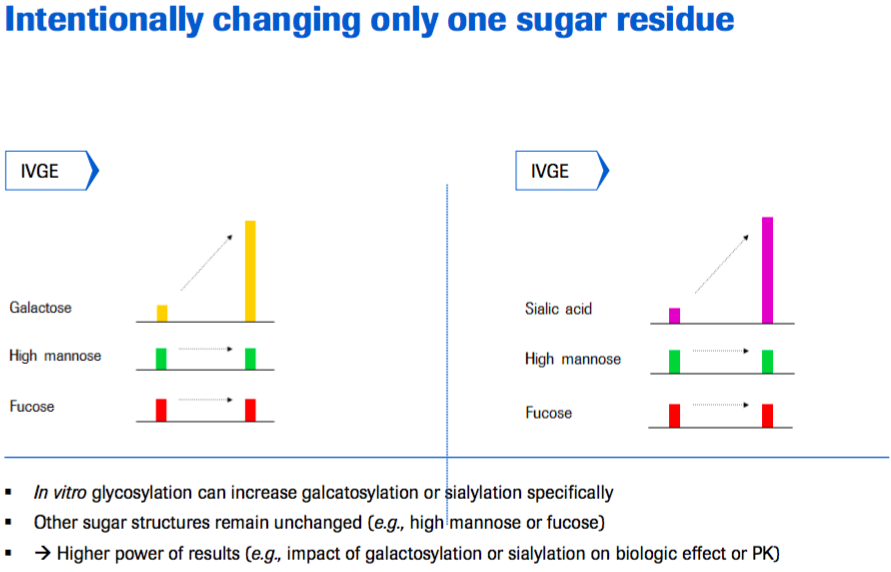
The first issue Mr. Dorn addressed was whether you can substantially change/control the glycosylation – does it work? Mr. Dorn stated that using IVGE technology you can build a glycopattern of choice by selecting the appropriate enzyme and substrate combination. For example, he presented data to show a substantial change in the glycosylation, by demonstrating a significant increase in galactosylation (Figure 2) and sialylation (Figure 3). In figure 2, the starting material is degalactosylated and within 8-24 hours they have achieved 100% biantennary galactosylation of IgG using galactosyltransferase.
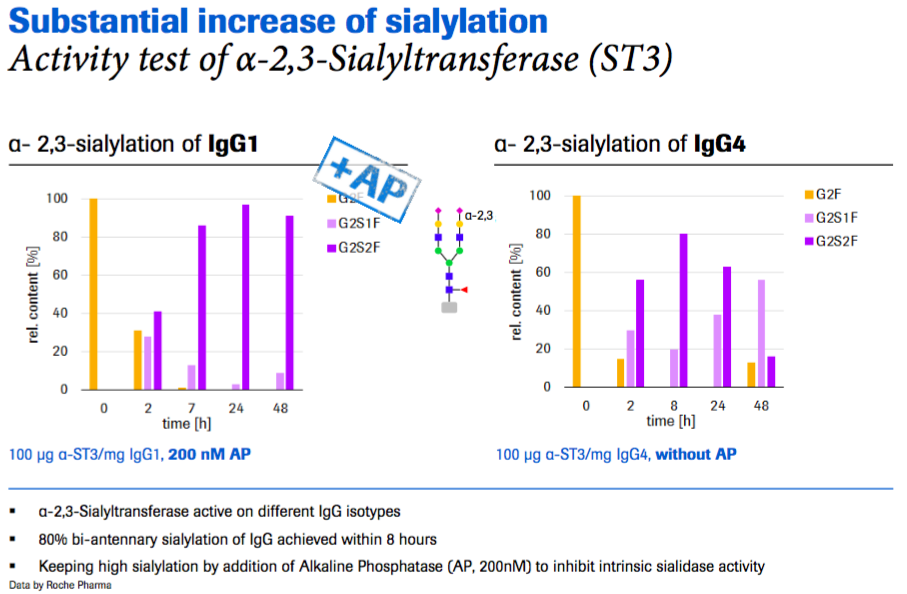
In figure 3, the starting material is desialylated and within 8 hours they have achieved 80% bi-antennary sialylation of the IgG using sialyltransferase. You can also see that after longer incubation times the sialylation begins to decrease due to an intrinsic feature of the sialyltransferase, but this effect can be inhibited by adding alkaline phosphatase (AP) to inhibit the sialidase activity, so sialylation levels remain high (as shown in the left side of the figure).
Versatility
The next area Mr. Dorn addressed was the versatility of IVGE and whether it can be applied to various targets. Mr. Dorn was able to share that activity on different IgG subclasses has been demonstrated, although enzyme kinetics may vary by target protein. Thus, it is best to optimize the reaction conditions for each individual application.
Pharma Requirements for Raw Materials
Lastly Mr. Dorn discussed the importance of having IVGE meet the requirements for drug manufacturing. Some key areas to consider include that the raw materials should ideally be antibiotic free, animal free, available in large scales, and must meet GMP conditions. IVGE raw materials meet all these requirements, GMP on request.
In Vitro Glycoengineering Implementation
Next, Dr. Marco Thomann, Manager Pharma Technical Development Analytics, Roche Diagnostics walked through how to set up a IVGE reaction and shared additional data on the benefits.
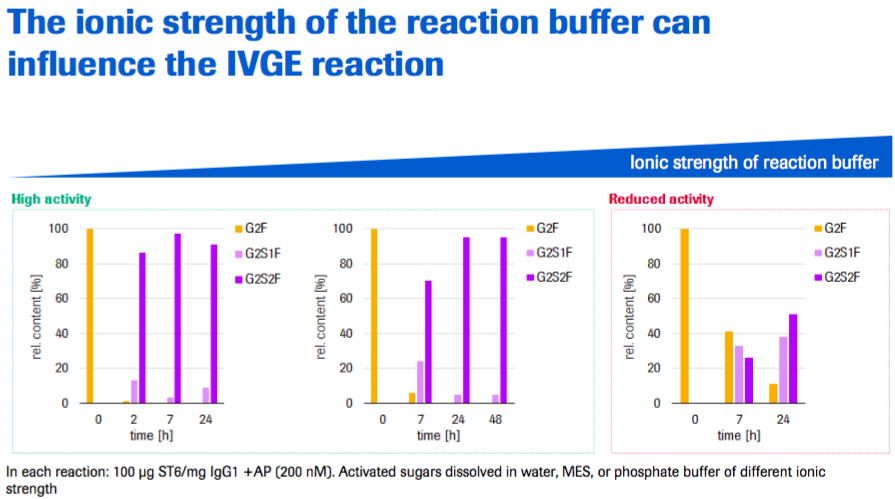
Dr. Thomann began by stating that setting up an IVGE reaction is relatively easy and straight forward. One note was that the ionic strength of the reaction buffer can influence the IVGE reaction. The enzymes are active in different reaction buffer compositions up to a certain ionic strength. His recommendation was to dissolve the lyophilized activated sugars in water, instead of buffer (Figure 4).
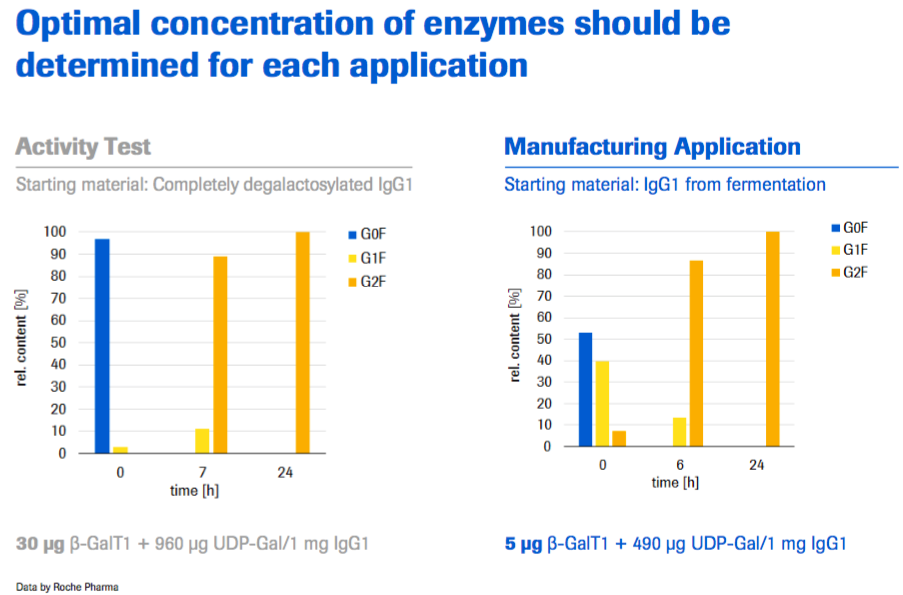
In addition, optimal Concentration of enzymes should be determined for each application as was also mentioned by Mr. Dorn (Figure 5).
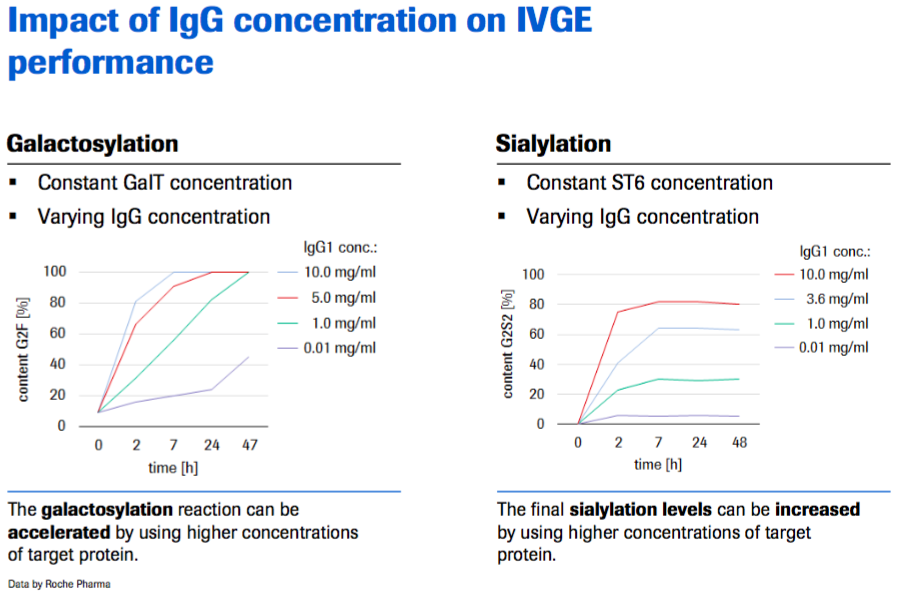
Dr. Thomann explained that IVGE performance is impacted by the IgG concentration. The galactosylation can be accelerated using higher concentrations of the target protein. Final sialylation levels can also be increased by using higher concentrations of the target protein (Figure 6).
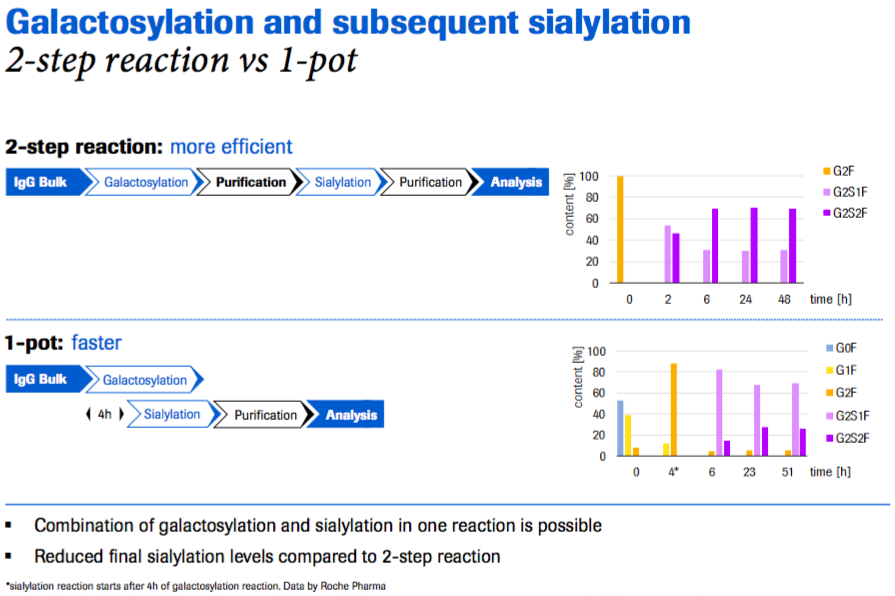
Next Dr. Thomann walked through their determination of where IVGE best fits into the manufacturing process. Roche tested both a two step and one step reaction. What they found is that the best option depends on what a user wants to achieve. The combination of galactosylation and sialylation in one reaction is possible and it is faster, but it results in reduced final sialylation levels compared to the two step reaction (Figure 7).
Next they looked at how to avoid additional purification steps in downstream purification. They tested conducting the IVGE on supernatant after harvest, on material that had completed a Protein A step, and lastly on the drug substance that has gone through the complete purification process. What they found is that conducting IVGE after harvest is too early and isn’t efficient. However it is possible to conduct IVGE after the first Protein A purification step and the results were similar to conducting it using fully purified material (Figure 8).
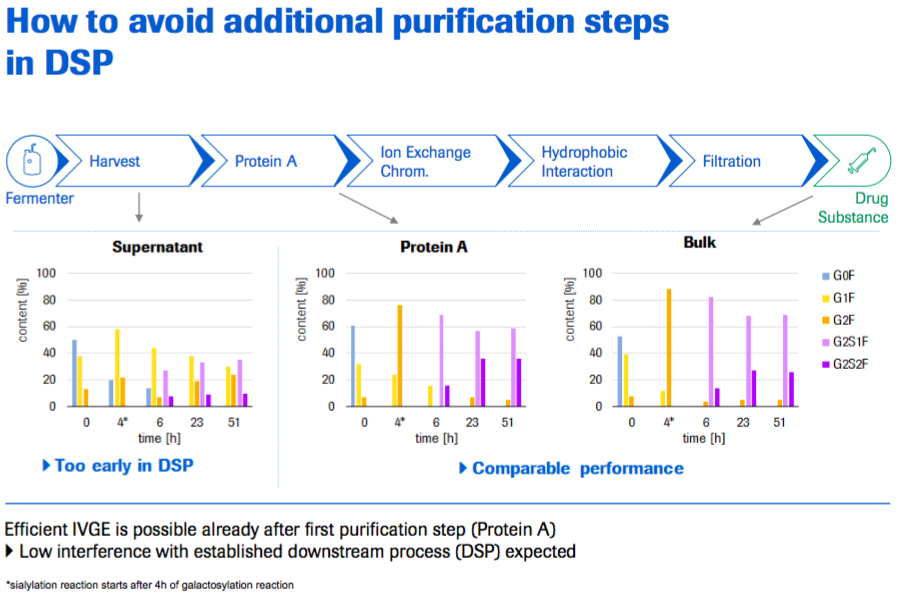
The advantage to conducting the IVGE reaction during the downstream purification process is that the introduced reagents are subject to the successive purification steps of regular downstream purification. Marco noted that Roche has residual assays in development to measure glycosyltransferase levels in the final drug substance. These assays can therefore be used to improve downstream purification development and to facilitate communications with authorities.
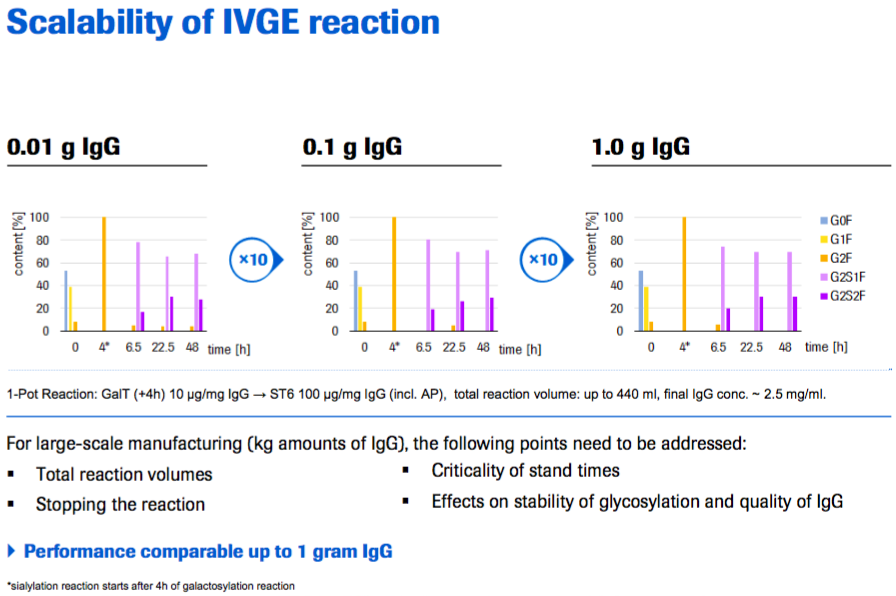
Next Marco discussed Roche’s testing of the scalability of IVGE. In the scales they tested (up to 1 gram of IgG (Figure 9)), the performance was comparable. For large scale manufacturing (kg amounts of IgG), the following points still need to be addressed:
- Total reaction volumes
- Stopping the reaction
- Criticality of stand times
- Effects on stability of glycosylation and quality of IgG
Dr. Thomann then presented three Case Studies that were very informative:
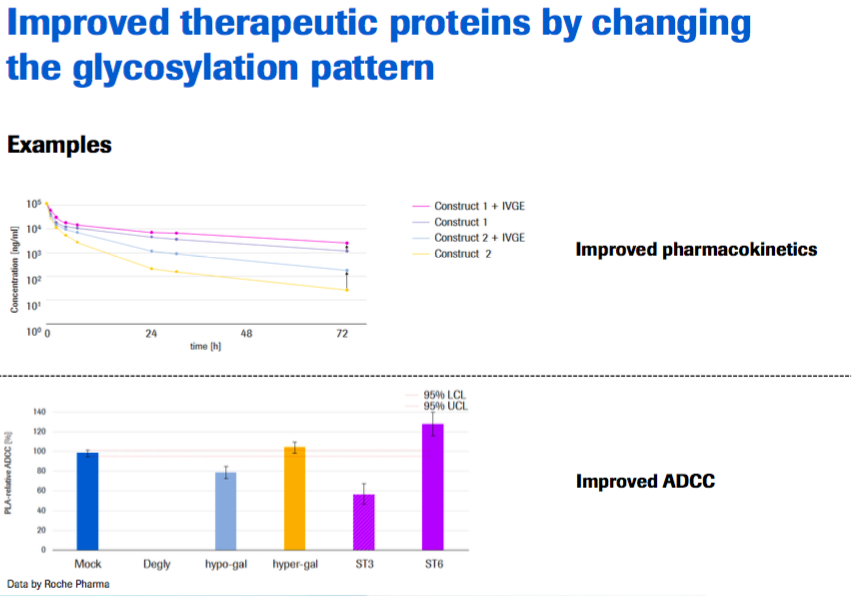
- Case Study I focused on structure-function analysis and demonstrating that using IGVE the effect of different glycosylation patterns on receptor binding and biologic effects e.g. ADCC (Antibody-dependent cellular cytotoxicity) could be investigated in an efficient way.
- Case study II examined the effect of glycan variants on pharmacokinetics.
- Case study III provided support of critical quality assessment and comparability studies.
Key Advantages of IVGE
In summary, Mr. Thomann explained that so far Roche Pharma sees that IVGE adds key value in in the following areas:
- CQA (Critical Quality Attributes) assessment
- Comparability studies
- Structure – function analysis
- Pre-clinical studies (PK, Tox)
Mr. Dorn then summarized the key benefits of IVGE:
Besides the reduced efforts to produce reference material and increased power of results in analytics, there seems to be potential to improve therapeutic proteins by changing their glycosylation pattern.
- More control over glycosylation in the manufacturing process
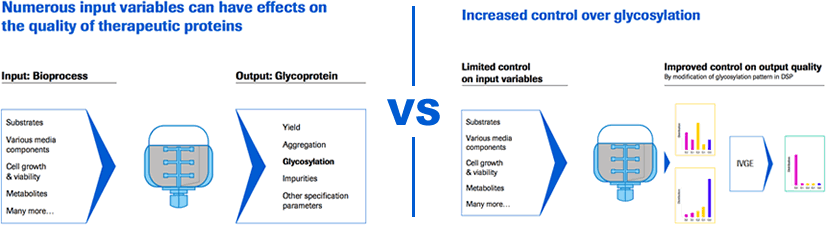
- Shifting a certain extend of glycosylation control to DSP, thereby enabling the USP to have more space to focus on other parameters, e.g. yield
Increased power of results
- High degree of galactosylation and sialylation achieved
- No significant influence on other glycoforms and other post-translational modifications.
To learn more, please view the webinar in full:
Products are for further processing only.