GMP-Compliant Benzonase® Endonuclease Safety Plus Emprove® Expert Provides Enhanced Risk Mitigation
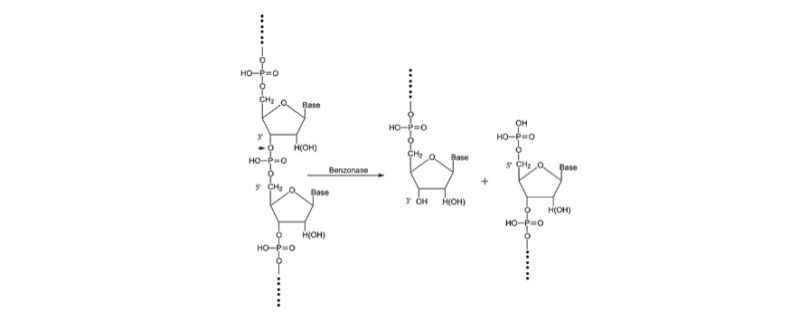
Benzonase® endonuclease has been used in biopharmaceutical production for DNA removal for over 30 years. It is a genetically engineered endonuclease, from Serratia marcescens, which can completely hydrolyze all forms of DNA and RNA (single-stranded, double-stranded, linear, and circular) into smaller oligonucleotides (<6 kDa) with no base preference. The use of Benzonase® endonuclease for downstream virus purification improving speed of purification, protects the downstream chromatography and filter devices from fouling, and give better product purity and yield while also meeting regulatory requirements.
The new Benzonase® endonuclease Safety Plus Emprove® Expert adds a GMP-compliant product to MilliporeSigma’s existing Benzonase® endonuclease portfolio. This product delivers enhanced risk mitigation above the standard Benzonase® endonuclease. It is manufactured in accordance with stringent quality standards and safety guidelines along with extensive documentation and enhanced product release testing. Additionally, the utilization of a chemically defined fermentation medium for manufacturing eliminates the variability and potential contamination risks associated with animal-derived media.
With these new features, Benzonase® endonuclease Safety Plus Emprove® Expert is ideally suited for manufacturing of cell and gene therapy agents such as adeno-associated and lentiviruses, oncolytic viruses, and viral vector vaccines.
Key Features
- GMP compliant manufacturing standards (ICH, Q7)
- Utilizes a origin chemically defined fermentation medium
- Additional quality testing to ensure absence of mycoplasma and adventitious viruses
- Tailgate samples facilitate incoming good control for large pack sizes adds a further layer of product safety
- Temperature strips on packages to monitor cold chain during shipping
Since Benzonase® endonuclease products share the same amino acid sequence (proven by LC-MS/MS mass spectrometry), robustness and activity, there should be no need to re-qualify your process when switching from Benzonase® endonuclease Emprove® Expert to Benzonase® endonuclease Safety Plus Emprove® Expert making it easy to incorporate into your workflow. (This regulatory assessment represents a general view of MilliporeSigma. It remains the obligation of the end user to perform a risk assessment for the respective product and to align it with the respective regulatory authorities).
| Charactoristics | Standard Benzonase® endonuclease EMPROVE® Expert | New Benzonase® endonuclease Safety Plus EMPROVE® Expert |
|---|---|---|
| Origin | Serratia marcescens, Production: E. coli K12 strain W3110; 30 kDa; PI 6.85; sequence homology (LC-MS/MS) | |
| Non-Animal-Origin (NAO), expressed in E.coli in chemically defined fermentation medium | No | Yes (animal-origin-free) |
| Lot release in vitro test for absence of adventitious viruses (3 cell lines) and Mycoplasma test | No | Yes |
| Lot release testing for Mycoplasma | No | Yes |
| Tailgate samples | No | Yes (with 5M unit size) |
| Shipment with temperature strips | No | Yes |
| Microbial testing | < 10 CFU/100,000 U | < 10 CFU/100,000 U |
| Endotoxins (LAL) | < 0.25 EU/1,000 U | < 0.25 EU/1,000 U |
| GMP manufacturing according to ICH Q7 | Yes | Yes |
| FDA Bulk Biological Master File (BBMF) | Yes | Yes |
| Long term product availability | Both Benzonase® products will remain in our portfolio | |