
Hollow Fiber Provides a Sweet Spot for Several Biomanufacturing Applications
Introduction
In the last five years or so, the biopharmaceutical industry has been increasingly discussing the benefits of single-use products, closed systems, continuous processing and 3D cell culture, meanwhile hollow fiber culture has been employing all these attributes for over four decades.
The use of hollow fiber technology for cell culture began in 1972 when Richard Knazek, et. al. published in Science, “Cell culture on artificial capillaries: an approach to tissue growth in vitro.” In the paper they describe the creation of a culture system with artificial capillaries that can be perfused with medium and where cells can attain “tissue-like densities in vitro.” Products are then secreted into the medium allowing for harvest of product without impacting the entire culture.
Since the 1980’s hollow fiber has been a popular choice in manufacturing products for the in vitro diagnostics market and was also used for the clinical manufacturing of whole cells (1, 2). Despite proven success in monoclonal antibody manufacturing, the use of hollow fiber bioreactors has not reached widespread use in biopharmaceutical manufacturing – yet. Evolving demands and trends in biomanufacturing coupled with improvements in hollow fiber bioreactor technology has created an opportunity for hollow fiber to provide unique advantages in biopharmaceutical, Cell Therapy, and vaccine manufacturing.
How Hollow Fiber Technology Works
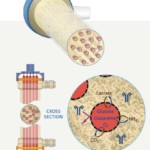
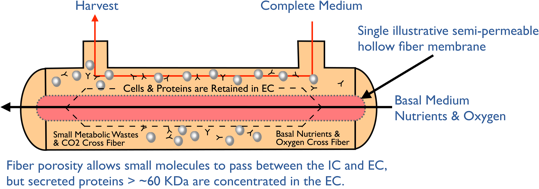
Hollow fiber perfusion bioreactors contain thousands of tube-shaped, semipermeable capillary membranes called hollow fibers. The hollow fibers are packed together in a cylindrical cartridge with an inlet at one end of the cartridge and an outlet at the other (Figure 1). The fibers are packed in a way that allows cells to be seeded and grown in the extracapillary space (ECS) outside the fibers. Medium continuously flows through the inside of the hollow fiber in the intracapillary space (ICS). By utilizing the hollow fiber membrane’s molecular weight cut off, typically between 10-100 kDa, you can control the flow of molecules. Thus, small molecule nutrients and oxygen are able to flow through the fibers and be delivered to the cells, while waste travels across the fibers to the intracapillary space to flow out. Importantly, the molecular weight cut off keeps the larger molecular weight products including monoclonal antibodies, recombinant proteins, viruses or whole cells to be held within the extracapillary space, (Figure 2). Spent media can then be removed and replaced with fresh media or oxygenated and returned. The flow of nutrients and waste in and out of the cells paired with the 3D culture configuration creates a considerably more in vivo-like environment, allowing the growth of cells to very high densities, in the range of 10e9 cells/ml.
Hollow fiber culture is a continuous perfusion process with typical runs being 60-180 days and product can be obtained through continuous or batch harvests.
Cell Culture Benefits
There are several advantages of the hollow fiber bioreactor system that allow for enhanced cell growth. Perhaps most prominent is that cells are allowed to grow in 3D culture at very high densities, which provides more in vivo-like conditions and healthier cells. From research with 3D culture models in comparison to 2D, we know that 3D cell culture allows cells to regulate their life cycle and maintain homeostasis, functions that may be lost in a 2D environment. While we have developed technologies to improve cell health and viability in 2D culture, without the 3D architecture these cells have significant differences when compared to 3D cell culture models and native tissue, including cell morphology, viability, cell growth rate, cell function, and expression levels (3).
A more native like environment, with higher cell density and increased cell viability, results in a reduction of apoptosis in culture. In hollow fiber bioreactors there is also significantly less shear stress, which can also cause apoptosis. A reduction in apoptosis decreases the amount of host cell proteins found in product harvest, increases product quality, and allows for a simpler downstream purification process.
The sum result of these culture conditions is that the cells can maintain high cell density and viability for extended periods, which leads to very consistent culture and product harvest.
Economic Benefits
Perfusion culture in general continues to gain ground in specific applications due to the economic benefits and manufacturing flexibility. Perfusion culture systems, including hollow fiber bioreactors, offer several advantages over traditional fed-batch bioreactors when addressing problems of scalability and increasing demand. Maybe the most critical advantage is that perfusion bioreactors are smaller in size and can produce the same product yield in less space. Perfusion bioreactors operate at 10-30x concentrations compared to fed-batch bioreactors. Hollow fiber bioreactors concentrate the product even further with concentrations of up to 100x, which simplifies downstream processing and lowers associated costs.
For example, it has been shown that a hollow fiber bioreactor, Biovest’s MAXIMIZER designed to replace roller bottles, ascites and stirred tanks, can produce 10-30 grams of monoclonal antibody per month in non-optimized cell lines using only about 5 square feet of bench space. For larger volume manufacturing needs, the yield of their XCELLERATOR, at only 12 square feet, is the equivalent to stirred tank bioreactors at the 1650-L scale, making hollow fiber a suitable platform for producing 10’s of kilograms annually. These yields are accomplished by adding additional units, as necessary, where the risk of losing one run affects only a fraction of the total production capacity. Operating multiple, linearly scalable units has the additional economic benefit of giving the manufacturing line the flexibility to move between single, large productions to several smaller ones – an approach not possible with large, fixed-volume stirred systems. Due to decreased media supplementation costs and increased automation, in both systems it is estimated that cost per gram of antibody can be reduced from $300 or more per gram in traditional manufacturing systems to less than $65 per gram using hollow fiber.
Additionally, due to better cell line engineering techniques, cell lines with much higher titers are now capable of producing biopharma’s hundreds of kg quantities in relatively small tanks (1000-3000 liters). As a result the days of 10,000 and 20,000 liter tanks are going by the wayside and much smaller tanks 1000-3000 are now being selected to handle manufacturing for these large demands. Thus the 1650 liter tank equivalent Xcellerator and other similar hollow fiber systems could be chosen to address manufacturing demands at these levels as well. Additional economic benefits include:
Time and Labor Reduction
- Easy set up – the bioreactor equipment has a small footprint and relatively easy set up. There is no need for specialized facility preparation or extensive equipment installation.
- Seed train not necessary – once cells have been scaled up to 0.5-3 liters of cells they can be injected directly into the hollow fiber bioreactor EC space where they will continue to expand and grow to high densities.
- No adaptation – the system can support either adherent or suspension cells, so there is no need to adapt cells to suspension, which can be a time consuming and challenging process.
- Automation – most hollow fiber bioreactors support automation, which greatly reduces daily maintenance in comparison with other manufacturing systems. Most systems require only 20-30 minutes daily due to automated monitoring and control of pH, gassing, temperature, lactic acid levels and rate of nutrient delivery.
- Single-use – disposable cartridges minimize cleaning/validation time and product change over time in cases where multiple products are being manufactured in the same facility. The fact that the cells are maintained for 60-120 days also decreases the amount of downtime between runs.
- Simplified downstream purification – because of the high cell density and the confinement of cells to the extracellular space, product concentration of 100x or higher can be achieved and because of the reduction in apoptosis there is little host cell proteins to contaminate product harvest. All of these factors result in downstream purification that is simplified and more cost effective. Some hollow fiber bioreactors even offer a built in, automated clarification step to further reduce downstream purification efforts.
Reduced Capital Investment and Operating Costs
The footprint of these bioreactors is very small considering output and come in a range of sizes to accommodate varying product demands. In addition to the space benefits, these systems are essentially plug and play and don’t require special facility configurations. Most systems can be operational with as little as a 100-240 VAC outlet, a CO2 source, and a network cable. This size advantage is important because it means that facilities don’t need a significant increase in space to increase production. Similarly, these bioreactors reduce utility costs and they are less labor intensive to operate. Thus requiring significantly less capital investment on the front end and lower operating costs to manufacture the same yield as fed-batch bioreactors.
Another advantage is that hollow fiber technology is very scalable. Additional product can be manufactured by simply extending run times or by adding or moving additional equipment from another production line. Once the process requirements for one cartridge are determined during process development, the process scales linearly, quickly, and predictably based on higher numbers of like cartridges in use. While the size of the hollow fiber cartridges themselves don’t change, additional cartridges can be added in parallel to increase scale. If demand decreases, run times can be shortened and equipment can be taken off-line or moved to another project.
While some have stated that perfusion culture can be more costly in terms of consumables, due to the continual flow of media needed, these costs are frequently offset by other savings. One nice feature of hollow fiber bioreactors is that, in most cases, a simple basal media containing no growth factors or serum is sufficient and can be used to flow through the intracapillary space with more complex media only added to the smaller extracapillary space. Media can also be re-circulated after oxygenation, reducing the overall amount of media needed, thereby reducing media costs overall.
Enables a Flexible Facility
The fact that hollow fiber bioreactors operate using a small footprint and are a closed system makes them a good fit for a flexible facility. Some advantages of flexible facilities include:
- Smaller facilities with a simpler design that can be duplicated in multiple locations
- Multiple products can be manufactured in the same facility or space
- Less segregation
- Equipment can be moved around on skids as needed to meet product demand in multiple production lines
In addition, hollow fiber bioreactors are a flexible manufacturing system with success in most mammalian cell lines including CHO, hybridoma, NSO, HEK-293, and others. There has also been success using insect cells and avian cells.
Reduction of Risk
Operating in a closed system means reduced risk of contamination. One of the biggest benefits of implementing a closed system, whether in research, pilot, or large scale, is in reducing the risk of contamination by viruses or other adventitious agents. Open systems naturally provide more opportunities for contamination because the process is open to the room environment and handling by operators. The automation of hollow fiber bioreactors also leads to decreased operator handling, which is important in decreasing contamination risk.
There are also safety concerns associated with breeches of product containment. Operations like fluid transfer present a much higher risk in an open system where splashing and lost media can occur. A closed system, by design, provides physical barriers to reduce the risk of contamination and contain the product. This is important because contamination can be extremely costly, not only in product loss, but also facility shut downs, cleaning, and validation.
Manufacturing Applications
Diagnostics Industry
The diagnostic market uses hollow fiber technology for a good deal of its production. It is a good fit for the industry because hollow fiber bioreactors can deliver on product consistency and a low cost of goods for an industry where the range for cost of goods is tight. In addition, the scale is a good fit for hollow fiber with product needs in the gram to one-kilogram range.
Mouse ascites are also used to manufacture antibodies for the diagnostic industry. Hollow fiber bioreactors provide many benefits over mouse ascites. Firstly, manufacturing in mice causes pain and distress to the animals, which is unnecessary in most cases, especially considering there are more efficient and scalable alternatives. This type of manufacturing also is labor intensive, requires a large facility and is not easily scalable. There are also contamination risks and more extensive downstream processing required when using mice. Lastly there is a great deal of variability in both titer and quality. Due to these issues, many companies are choosing to move away from this type of manufacturing. Furthermore, several European countries have imposed full or partial bans on mouse ascites manufacturing, making hollow fiber bioreactors a great alternative.
Monoclonal Antibody and Recombinant Protein Manufacturing
Hollow fiber bioreactors are also a good fit for monoclonal antibody and recombinant protein manufacturing, particularly in light of increasing interest in flexible, multi-product facilities. While hollow fiber bioreactor systems are an option for large volume product manufacturing, stirred tanks may still be a better, more cost effective choice. Yet there are some smaller applications in the gram to 1 kilogram range that make very good candidates for the use of hollow fiber bioreactors. Some areas to consider:
- FDA Licensed in vivo imaging agent – ProstaScint® (NDC:57902-817-01) has been on the market since October 28, 1996 and is manufactured using Biovest’s hollow fiber bioreactor systems. This drug is still actively used in the market place as a diagnostic imaging agent in newly-diagnosed patients with biopsy-proven prostate cancer, thought to be clinically-localized after standard diagnostic evaluation (e.g. chest x-ray, bone scan, CT scan, or MRI), who are at high-risk for pelvic lymph node metastases (seeCLINICAL PHARMACOLOGY, Imaging Performance in Newly-Diagnosed Patients). It is not indicated in patients who are not at high risk.
- Clinical material – often in preparation for Phase I and II clinical studies there is a need to manufacture clinical study material without the proven clinical results to justify large capital or process development expenses. Time is also a key factor as product manufacturing can sometimes be a challenge to moving quickly into the clinic. Hollow fiber bioreactors can offer an attractive option. As discussed earlier, these systems are relatively easy to set up and with no need for seed train expansion; they are a quick, cost effective way to get material quickly. They are also easily transitioned to a traditional stirred tank bioreactor if studies are successful and high product volume is required.
- Multi-use Facilities – another opportunity for hollow fiber bioreactors is in instances where high flexibility is required. The small footprint, single-use, and closed system attributes make it a good fit for multi-product or multi-use facilities. In particular Contract Development and Manufacturing Organizations (CDMOs) could make use of this technology as their facilities are routinely being used for many different products.
- Low volume therapeutics – low volume therapeutics is also an area that could benefit from the use of hollow fiber bioreactors. Orphan drugs or high potency drugs often have little need for large volume (many kilograms) manufacturing. The scalability of the hollow fiber bioreactor system could offer a convenient way to manufacture material while still being able to quickly address any changes in product demand.
Emerging Manufacturing Applications
Just as hollow fiber bioreactors have been proven in certain areas, there are other emerging areas where the technology could be a very good fit. Some examples include:
- Viral vaccines – cell lines used for vaccine manufacturing can be particularly tricky and usually require a more robust culture system for healthy culture. Due to the more in vivo-like state found in hollow fiber bioreactors, the system can address the needs of many cell types including mammalian, insect, and avian cells used in vaccine manufacturing.
- Cell Therapies – with the characteristics of high cell density and in vivo like environment, hollow fiber could be a great system for manufacturing cell therapies. As mentioned earlier, this technology has been used to successfully manufacture whole cells for Cell Therapy in the past and as the Cell Therapy industry advances, efficient, cost effective ways to manufacture large numbers of cells will be needed.
- Cancer treatments – new cancer treatments, including cancer vaccines and T cell therapies, also use whole cells. Hollow fiber bioreactors could prove to be a good system for this kind of manufacturing as well.
- Disease Modeling – because of the native like tissue state of the cells in hollow fiber, the system could be used to model disease. One example can be found in the study, “Co-culture of Stromal & Erytholeukemia Cells in a Perfused Hollow Fiber Bioreactor System as an In Vitro Bone Marrow Model for Myeloid Leukemia,” published in Biotechnology and Bioengineering, (Usuludin et al., 2012). The authors describe how they developed a hematopoietic co-culture system using Fibercell Systems Hollow Fiber Bioreactor. By using the hollow fiber bioreactor, study authors were able to create a culture environment that more closely represented human bone marrow. The ability to achieve this superior model was due largely to the capability of the hollow fiber bioreactor to support high cell densities and 3D cell culture, thus allowing cells to behave in a way that was more similar to natural cell interactions. In addition, the use of the hollow fiber bioreactor with perfusion permitted culture to continue for two weeks as compared with only one week using standard tissue culture techniques. Another example of disease modeling can be found in the paper, “Human hepatocyte functions in a crossed hollow fiber membrane bioreactor,” where study authors used hollow fiber to create a liver model that could potentially be used in both disease modeling and drug metabolism or toxicity studies.
Conclusions
The evolving biomanufacturing landscape has created an opening for manufacturing processes that are more scalable and flexible with reduced capital investment and operating costs. In exploring hollow fiber bioreactors, I found that there are many potential applications for this technology. While in most cases the large volume manufacturing of monoclonal antibodies (hundreds of kilograms) may still be best suited for stirred tank bioreactors, there are several products and manufacturing scenarios that could benefit from the advantages, flexibility and capabilities of fully automated hollow fiber bioreactor systems.
References:
- Melink, G. B., Method of Culturing Leukocytes. U.S. Patent 5,541,105, July 30, 1996
- Zarling, J. M., Method of Adoptive Immuotherapy for Treatment of AIDS. U.S. Patent 5,081,029, January 14, 1992, see column 8, line 30.
- “Materials and Assay Systems Used for 3D Cell Culture,” Cell Culture Dish