In-Process Analytics are Key to Successful Cell Therapy Process Optimization and Manufacturing
Cellular therapies are an increasingly viable therapeutic option for many indications, particularly for patients who have exhausted traditional treatments. Considerable clinical activity and the approval of several autologous, patient-specific therapies has increased industry demand and has also highlighted bottlenecks in manufacturing workflows. These bottlenecks must be addressed to improve manufacturing productivity, safety, and timely delivery to patients. In an autologous setting, each patient’s health status and demographics translates to a unique cell population, which imparts variability to the subsequent cell therapy manufacturing process. This emphasizes the importance of in-process controls in ensuring a consistent drug product, even in the presence of such variability.
One area of focus is process analytical technologies (PATs), essential to providing information on critical quality attributes (CQAs) throughout the manufacturing process and QC release testing. Real-time process feedback is essential for increased process monitoring and control to ensure manufacturing success, but often critical process feedback is delayed due to lengthy testing times. During the recent ASGCT virtual conference, there was an informative symposium focused on mass spectrometry (MS)-based strategies for in-process analytics to support cell therapy manufacturing development and optimization. Rich Rogers, Ph.D., from Bristol Myers Squibb (BMS) gave an excellent presentation outlining the analytical needs and instrument requirements for PAT and how MS-based PAT holds promise as a robust technique to overcome many of the challenges experienced with current analytical tools.
Attribute Monitoring for CAR-T Therapies
Dr. Rogers began by outlining the platform used at BMS for developing their autologous lentiviral-based CAR-T platform. In manufacturing autologous CAR-T therapies, the process begins with harvesting a patient’s cells using leukapheresis. Once the mononuclear cells are harvested, a number of steps are required to isolate, activate, engineer, and expand the T cells before the cell therapies can be reinfused back into the patient. Currently, BMS employs PAT to monitor cell proliferation (i.e., viability, density) and flow cytometry at each stage to perform T-cell and impurity profiling (i.e., presence of contaminating cell types). While the data from these tools are adequate, there are opportunities for more in-depth monitoring of cell health and metabolic status, which can affect the efficacy of the final product.
The possibilities for implementing PAT are endless and are applicable in every aspect of the manufacturing process. PAT can be used to identify process levers to ensure product consistency and to improve manufacturing success both upstream (i.e., temperature, pH, glucose, amino acids, cell viability, and metabolites) and downstream (i.e., process-related impurities) as well as for quantifying CQAs in the final product (i.e., purity, potency, safety, and CAR frequency).
Rich outlined some of the key features that make an instrument appropriate for in-process PAT:
- Instrument footprint: manufacturing suites often have limited space so instrument size is a consideration
- Capital investment: different cost considerations than instrumentation for R&D
- Number of bioassays: an instrument that can perform multi-attribute testing is preferred
- Low-volume sample requirements: especially during the manufacturing process, often times it is not possible to extract large volume samples for in-process testing
- Time to run the assay: time from sampling to data should be fast; turn-around time is critical to provide relevant process feedback
- Low technological expertise requirement: the training required to operate in-process instrumentation should be low, and be subject matter expert (SME)-free
- Speed to data: as short as possible/near real-time to have the biggest impact on manufacturing process
Mass Spectroscopy-based PAT Strategies
Rich then detailed both untargeted and targeted MS-based PAT strategies that BMS is developing for in-process analytics. MS is a well-established technique used to generate robust and reproducible data in large molecule therapeutics such as mAbs. However, applying this strategy to Cell Therapy involving thousands of proteins poses hurdles that need to be addressed. One hurdle is the complexity of the cells that are the basis of CAR-T therapies. One major difference between large molecule therapeutics and Cell therapy, is the mAbs are purified versus in cell therapy there are potentially thousands of proteins to consider. Therefore traditional large molecule MAM (Multiple Attribute Methodology) cannot be applied directly to Cell Therapy. There is a need for looking at specific attributes in PAT approaches for Cell therapy. The potential of this technique to provide more in-depth T cell profiling has led BMS to invest in MS-based proteomics and metabolomics approaches for MS-based cell therapy analytics.
Proteomics-based PAT
Rich then described the MS-based cell surface proteomics protocol utilized by BMS to produce an untargeted cell surface profiling of the T cells throughout all stages of manufacturing. Cell surface proteins are tagged with biotin, followed by cell lysis, streptavidin enrichment for the tags, enzymatic digestion, and MS analysis.
This untargeted approach is advantageous because it does not need reagents targeted specifically to a cell surface protein (unlike flow cytometry methods), which provides an unbiased, global view of T cell surface protein composition.
Process residual and secreted protein monitoring is another area where a multi-attribute targeted proteomics approach is being developed to replace ELISA, which is limited by the need to develop target-specific detection reagents. This MS method is highly sensitive, thus allowing for simultaneous, multiplex quantification of hundreds of protein targets. Targeted peptide selection from chromatography separation, fragmentation and Orbitrap MS analysis provides quantitative measurements of process residuals and T cell-secreted proteins.
In summarizing these proteomics approaches, Rich commented that while they are happy with the precision of the results, work is still needed for this method to meet the PAT requirements he outlined earlier in the presentation. MS instruments have a large footprint and can be expensive, personnel training is extensive and speed to data needs to be improved for providing timely information to manufacturing personnel during production runs in real-time.
Targeted Metabolomics-based PAT
In the second half of his presentation, Rich focused on targeted metabolomics-based PAT using the REBEL analyzer (908 Devices). Here the goal is to perform at-line metabolic profiling of the T cells by interrogating spent media from the bioreactors. Cell secretion and metabolite analysis can provide valuable information about the health and potency of the cells. BMS plans to implement the REBEL analyzer for metabolomics at every stage of process development. This includes the creation of the lentiviral vectors as well as in the T cell process itself where the REBEL analyzer has been used to provide valuable information on T cell health and metabolic status.
The REBEL analyzer meets all of the requirements for PAT:
- Small footprint (size of a microwave) and reasonable cost

- Monitors 30+ analytes simultaneously
- Requires only 10μl sample volume
- Pipetting skills are the only technical requirement
- Minimal training required
- Assay turnaround time is fast ~7 minutes/sample
- Rapid time to data: integrated software performs analysis and automatically generates a report that can be exported as a CSV or PDF files that is compatible with LIMS, PIMS
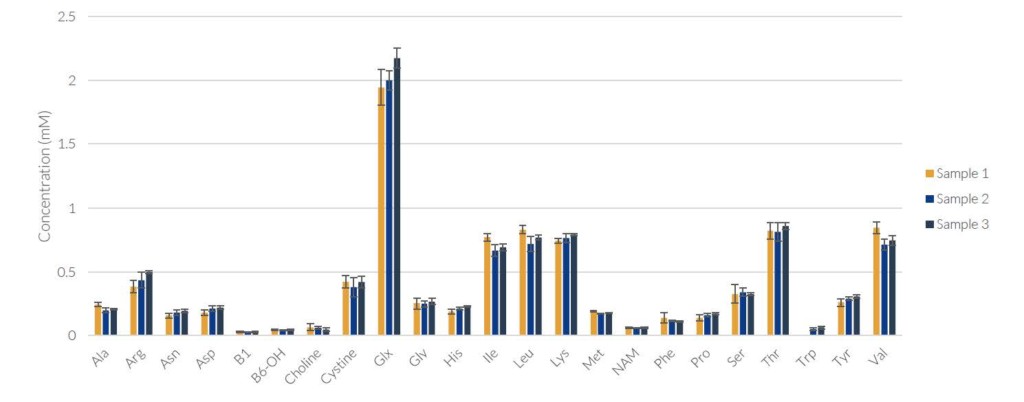
During method and instrument evaluation, the REBEL was used to run 3 separate samples from the same bioreactor run with 5X replicates to demonstrate high replicate reproducibility, shown below.
To close out his presentation, Rich stressed the importance of PAT to improve process understanding and manufacturing success for cell therapies where input cell variability is expected. Investment in PAT and innovative technologies are pushing the throughput and accuracy limits of current methods and Rich said that he sees great opportunities for MS-based methods in both targeted and untargeted proteomic and metabolomic characterization. Providing a broad spectrum, global assessment of the T cells throughout cell therapy manufacturing.
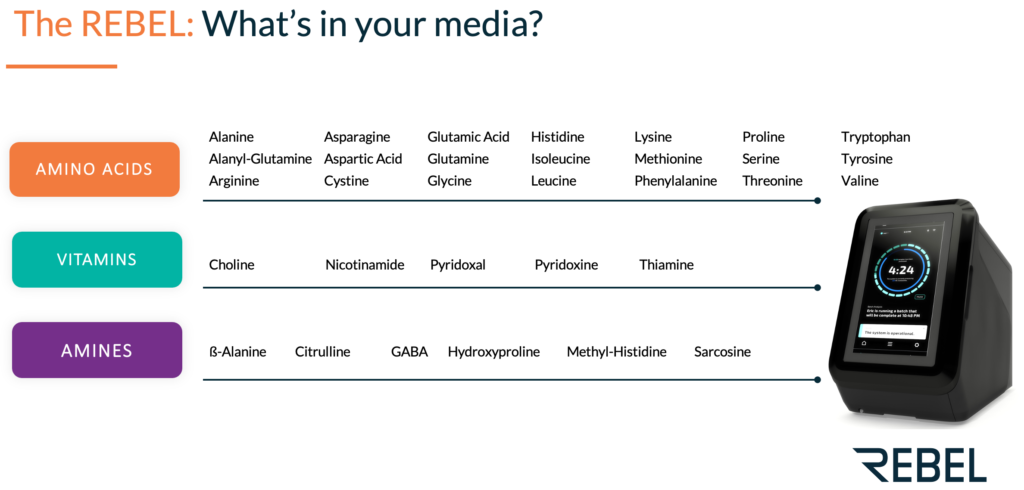
Following Rich’s presentation, Kerin Gregory, PhD, Product Manager at 908 Devices, gave a talk on how the REBEL analyzer can be used to achieve timely and useful process analytics through rapid nutrient monitoring of cell culture medium (i.e., amino acids, vitamins, amines). The cell culture medium provides key nutrients for cells cultured ex vivo and has a direct impact on cell growth, productivity and function of the subsequent therapeutic. In-depth media analysis can give insight into nutrient consumption to facilitate media optimization efforts to improve CQAs.
Nutrient Profiling and Media Optimization
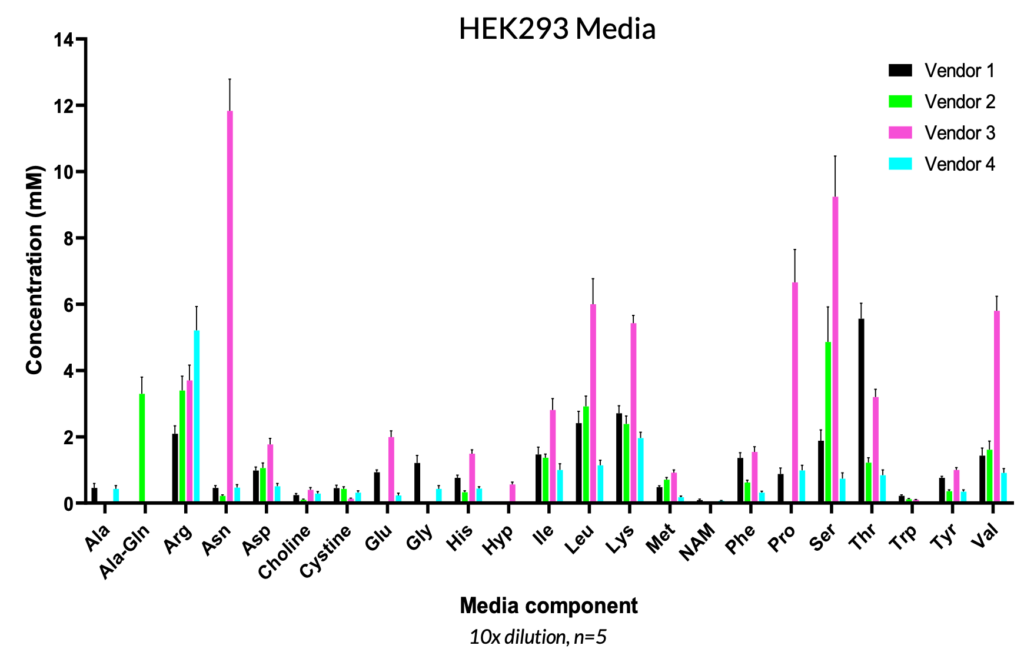
Specifically for viral vector manufacturing, the demand to meet clinical and commercial targets requires production of high viral titers. Nutrient profiling can provide a better understanding of host cell (i.e., HEK293) requirements and presents an opportunity for media optimization to improve viral titers, since clonal differences can impact cellular responses. While many commercial media formulations are available on the market, the relative concentration and composition can vary greatly, as shown below, and these various media options can have an important impact on viral vector titer and integrity. A. The REBEL can also be used to monitor lot-to-lot differences in selected formulations to ensure consistency and reproducibility.
Additionally, research has shown that T cell culture medium requires several key amino acids that modulate activation and expansion in vitro and they play a role in CAR-T cell metabolic readiness and anti-tumor efficacy upon introduction into the tumor microenvironment in vivo. This really underscores the profound effect that cell culture medium composition has on cell growth, metabolism, and productivity.
Serum Replacement
Kerin added that there has been a shift in the industry away from serum-containing medium formulations, which suffer from batch-to-batch variability and have potential contamination risk with adventitious agent in addition to immunogenic risk in clinical applications. The development of chemically defined formulations represents an area where the REBEL can be leveraged to characterize media for reformulation efforts. The REBEL has a dynamic range of 5-100uM to detect low levels of amino acids and other nutrients accurately. Analysis is not impacted by the presence of serum proteins, unlike other quantitative methods.
Overall, Rich and Kerin did a great job showcasing the need for instrumentation to further process development and PAT in-process monitoring. They demonstrated how the REBEL analyzer is being used to provide rapid, sensitive at-line measurements for spent media metabolite analysis, and for nutrient profiling during media optimization or reformulation for viral vector and CAR-T manufacturing.
To learn more about Process Analytical Technologies for Cell Therapies:
View the full presentation, ASGCT virtual conference – Mass Spectrometry-Based Process Analytical Technologies for Cell Therapies
Download the REBEL Cell and Gene Therapy Application Snapshot Booklet