In vivo-jetRNA® as a promising tool for RNA therapeutics
Nucleic acid-based vaccination is a promising technology that is particularly attractive to prevent or treat infectious diseases and cancer. A nucleic acid vaccine consists of a genetic sequence that is administered in vivo to be taken up by cells that will express at their cell surface or secrete the encoded antigen for induction of an immune response. Main technical challenges associated with the use of nucleic acid vaccines are efficacy and safety. To address these challenges, it is important to take into account the following parameters during initial research development of a genetic vaccine: the type of nucleic acid used and the delivery method.
Nucleic acid vaccines usually consist of engineered plasmid DNA and more recently also of messenger RNA sequences that encode for a specific antigen. While plasmid DNA is relatively inexpensive and easy to manufacture, their main drawback is efficacy. This is linked to the fact that plasmid DNA needs to be i) delivered into a tissue and ii) delivered into the cell nucleus for transcription and translation of the encoded antigen to occur. To improve delivery of naked plasmid DNA into tissues, cationic polymers have been developed to efficiently encapsulate it. in vivo-jetPEI® from Polyplus-transfection®, a linear polyethylenimine-based reagent, is currently the most successful, renowned and safe DNA transfection reagent suitable for in vivo delivery of DNA vaccines. in vivo-jetPEI® has been used in several DNA vaccination approaches, such as immunization against human papillomavirus, hepatitis B virus and HIV using several administration routes, respectively subcutaneous injection, intravenous injection and topical application (more details on in vivo transfection database).
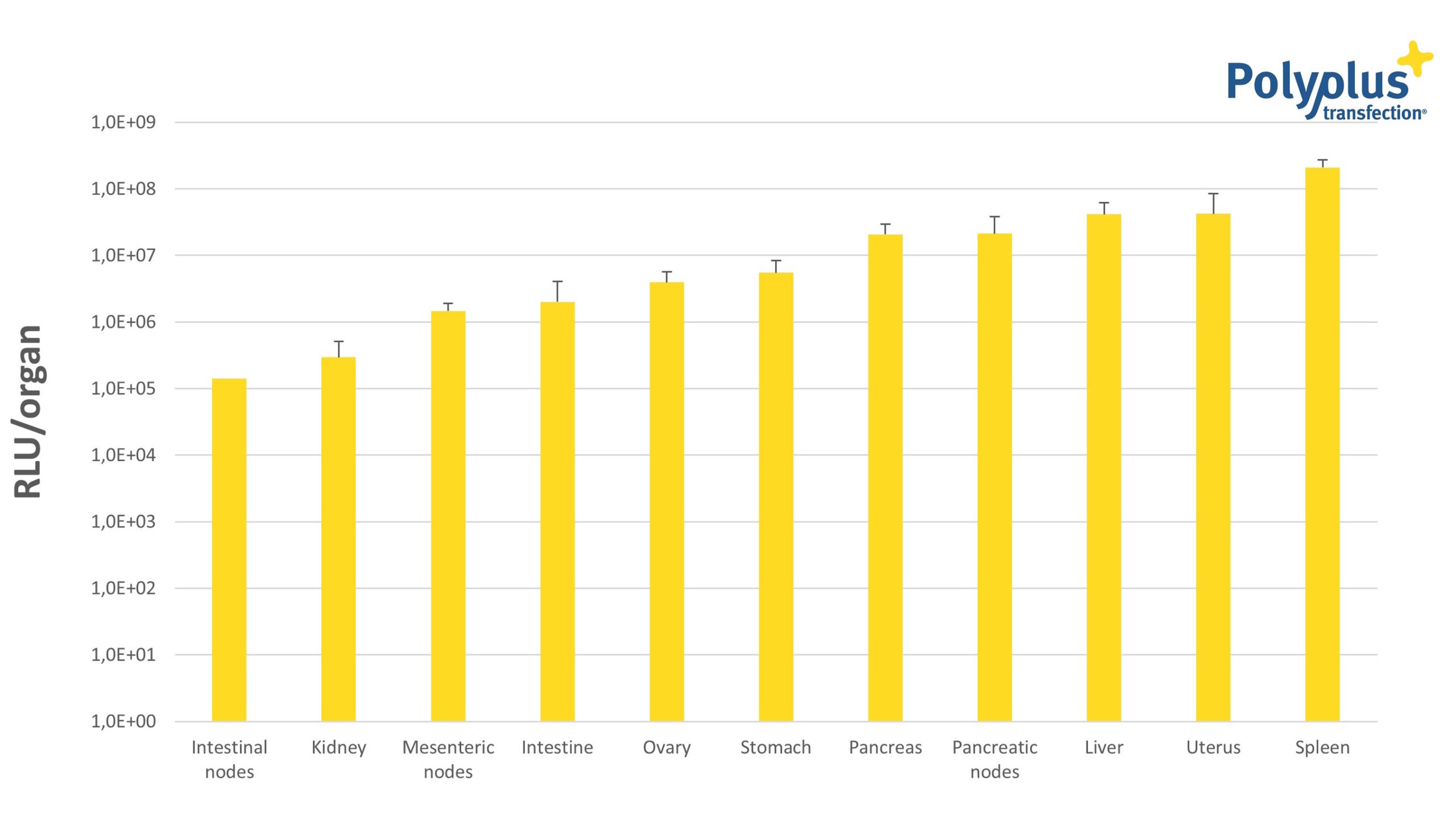
However, enhanced DNA vaccine delivery is not always sufficiently immunogenic. Therefore, messenger RNA-based vaccines have emerged as an alternative to DNA vaccination. As plasmid DNA, mRNA can be used to transiently express a specific antigen into an organism or tissue. The advantage of mRNA-based immunization relies on the self-adjuvant activity of mRNA and the fact that smaller amounts of nucleic acid are sufficient to obtain a robust immune response. The main drawback of mRNA is that it is not a stable molecule, hence the need for a transfection reagent that can efficiently protect mRNA during its delivery to cells, irrespective of the administration route used -systemic or local. in vivo-jetRNA® is a safe non-viral transfection reagent of choice that meets the above requirement with its properties: it protects its mRNA payload against endonucleases, prevents non-specific interactions with proteins and promotes efficient cell entry. in vivo-jetRNA®-mediated mRNA delivery leads to gene expression in numerous organs, including secondary lymphoid organs distributed throughout the body that play a major role in the immune response (Fig. 1).
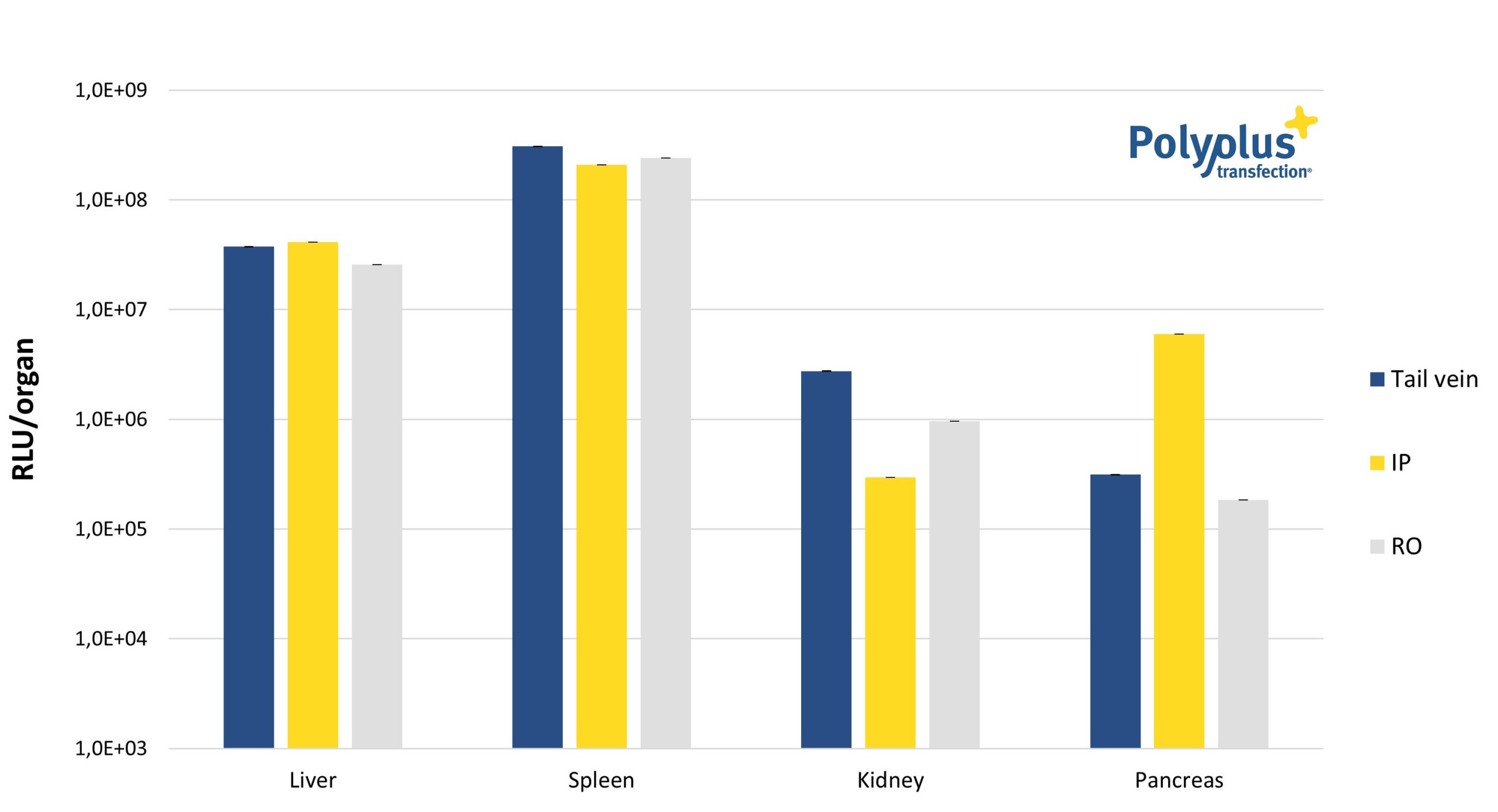
Furthermore, in vivo-jetRNA® is safely administered using most delivery routes, as targeted organs remain phenotypically intact and no tissue damage is observed post-injection (Fig.2). It is therefore an ideal candidate for additional applications, namely in vivo functional studies, immunotherapy and genome editing.