Industrial Scale Manufacturing of AAV: A Blueprint
The successful commercialization of adeno-associated viral vectors (AAV) relies on GMP-compliant and scalable, industrialized production platforms to rapidly advance high-quality products to market while also ensuring patient safety. The most utilized approach to AAV production is through the transient transfection of mammalian cell lines, such as HEK293 or derivatives, followed by viral vector harvest, downstream purification processes and analytics for final product testing. Since viral vector production is the most significant cost driver in AAV-based gene therapies, it is prudent to optimize each step of the GMP manufacturing process during the process development phase to ensure compliance of input materials, maximize yield and product quality while minimizing costs.
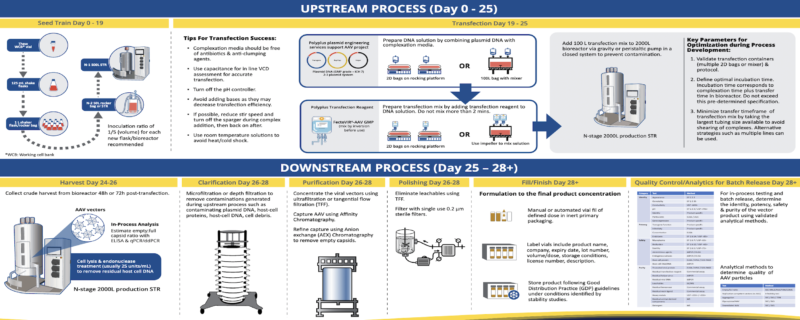
Polyplus has released an informative infographic that serves as a blueprint for industrialized AVV production. Based on a theoretical 2,000L bioreactor format, the infographic highlights the key upstream and downstream steps in workflow starting from seed train establishment all the way through to QC release testing criteria. As the industry’s leading transfection experts, Polyplus has also provided helpful tips for transfection success and important process parameters to optimize that are critical to productivity and quality of the AAV.
Implementing FectoVIR®-AAV GMP transfection reagent into the workflow can effectively improve scalability, productivity and flexibility for industrial manufacturing of AAV viral vectors in suspension cells. It is an animal free GMP grade transfection reagent manufactured in compliance with regulatory requirements (ICH7 GMP/ Eudralex Vol 4, Part II, Annex 1) for ancillary materials for maximum process robustness and process safety in large-scale workflows. Additionally, Polyplus offers plasmid engineering services to facilitate effective vector plasmid design that combined with the FectoVIR®-AAV GMP reagent can achieve reproducibly high titers of functional AAV.
To learn more about improving scalability, productivity and flexibility for industrial manufacturing of AAV viral vectors in suspension cells, please see FectoVIR®-AAV GMP transfection reagent