Innovations in Hybridoma Media
Hybridoma media is an interesting topic because there have been many advances in hybridoma media, especially lately. However, many scientists still use the same media formulations commonplace in the 1980’s, i.e. base media plus fetal bovine serum (FBS). I don’t believe it is a case of – “if it isn’t broke don’t fix it”. In fact, many have argued that a switch away from FBS is necessary as the harvesting and collection of FBS is an unnecessarily cruel treatment of animals. I would argue that the reasons FBS is a main element of many cell culture processes is that, until recently, there were few better options in terms of performance and reproducibility. Now with the recent introduction of new products that offer cost-effective, superior alternatives to FBS, the use of FBS or animal products is quickly becoming outdated technology in hybridoma production.
Recent estimates for the US hybridoma media market, not including FBS sales into this market, have reached $34 million dollars (USD). There are over 200 large and small companies in the United States alone who conduct research using hybridoma cell culture and NIH funding for academic accounts performing this work was estimated to be $19MM in 2011.
Base media plus FBS is the historical standard for hybridoma based antibody production. FBS provides a good source of nutrients, growth factors and many other ingredients that cells like. The problem is that FBS is a “black box” that contains many unknown, variable and undesirable ingredients also. Inconveniences of serum include the need to screen lots, variability in material cost and quality, the presence of contaminating IgG, and potential safety hazards in industrial applications. The need to screen lots of serum increases the final product cost and the presence of contaminating IgG has the potential to reduce the purity or quality of monoclonal antibodies. Animal-derived ingredients also carry the risk of introducing adventitious agents, such as viruses and prions that can contamination cell culture, the lab, and a production suite. Finally, objections have been raised to the use of serum in cell culture on regulatory and ethical grounds, and the undefined nature of FBS is increasingly being seen as a liability to researchers who need increased control over the growth of their cells.
As a result many have looked to serum-free media alternatives.
One alternative to using serum is to use base media plus animal-derived proteins or animal-based hydrolysates. Commonly included animal-derived proteins include bovine serum albumin, human plasma derived albumin, bovine transferrin, and human plasma derived albumin. While this is an improvement over FBS, these formulations still have issues with inconsistency and adventitious agent risk. In addition, pricing of these products over time can be variable due to fluctuating supply and demand.
Some companies offer serum-free, but not animal component free, hybridoma media. These media provide a serum free alternative, but not an animal free alternative. Companies including Life Techologies, Sigma-Aldrich and Thermo Fisher all offer versions of serum-free media for hybridoma culture that incorporate animal-derived serum proteins. The advantage is that these media provide an all in one solution and eliminate the work of optimizing media internally, while eliminating FBS. However, these media can result in a reduction in antibody yield when compared to serum and it can be challenging to transition cell lines to these media. Often lengthy cell adaptation times are required and many hybridomas fail to achieve robust growth. Due to this, many hybridoma labs have chosen to formulate their own serum-free media by either starting with a commercial serum-free media or an optimized base media such as DMEM/F12 and then adding key recombinant protein supplements.
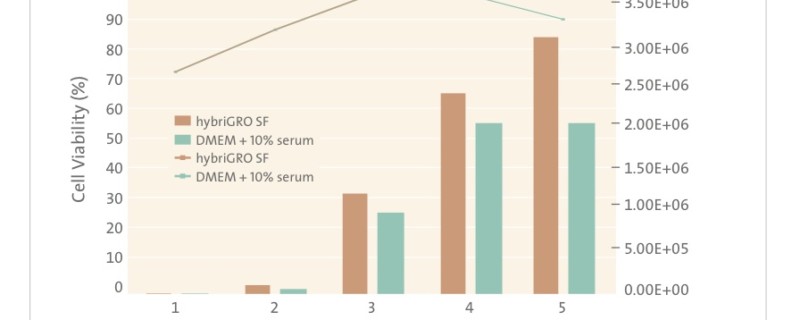
To ease the transition from serum and still maintain good antibody yield, serum-free media should be supplemented with products that help to replace some of the critical ingredients in serum. Innovative animal-free, recombinant supplements such as recombinant albumin and recombinant transferrin offer the ability to replace some of the key factors in serum involved in cell growth and viability. Since they are recombinant, they are also completely IgG free. Companies including Sigma, Fisher Scientific, InVitria, Sheffield Bioscience and Mediatech sell recombinant albumin and recombinant transferrin that can be added to serum-free media to provide an alternative to using serum in hybridoma culture. Corning Cellgro recently launched a unique new hybridoma medium called hybriGRO SF. Scientists interviewed by The Dish report that hybriGRO SF provides the high antibody titer they seek, yet not problems with bovine IgG contamination from FBS and no use of animal components in the media. It appears that Corning has made a breakthrough for their customers. This new product is animal component-free and defined and also contains low concentrations of highly purified animal origin free recombinant proteins in order to enhance cell growth and productivity. In addition, hybriGRO SF uses glutamine substitutes to improve medium stability and to reduce the generation of toxic ammonia by the cells [1]. Ammonia is a known inhibitor of cell growth and productivity. HybriGro SF represents a “best of both worlds” – an animal free medium already optimized with recombinant supplements. Recent data for hybriGRO suggests comparable growth and viability compared to that obtained by traditional serum supplemented cell culture media:
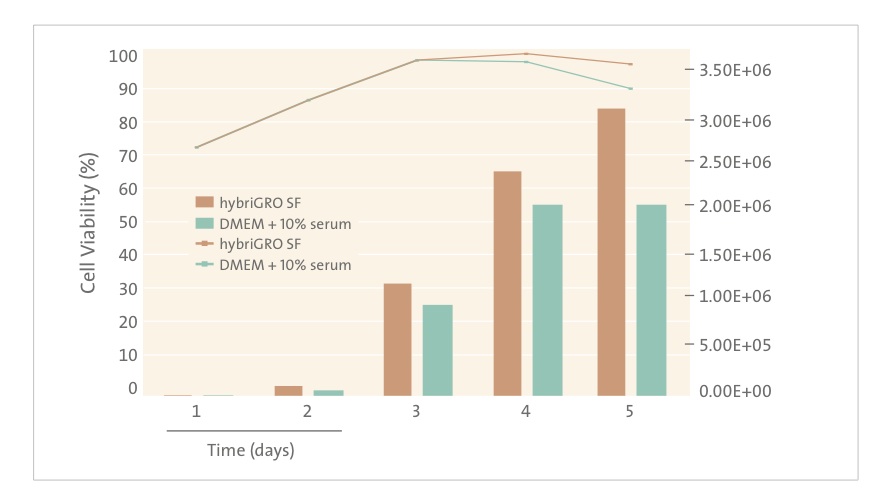
Animal-origin free (AOF) media eliminate the possibility that animal components are present in the finished product and provide more consistent performance. They facilitate the production of monoclonal antibodies consistently and efficiently using the best technology available. Now that cell growth and productivity with AOF media is comparable to or better than serum containing formulations, a switch to AOF is now more attractive than ever. Corning’s HybriGRO SF is the latest example of an advanced medium for this growing market that uses recent advances in cell culture technology.
Footnotes
-
1. Butler, M. and A. Christie, Adaptation of mammalian cells to non-ammoniagenic media. 1994. (7765956) Cytotechnology. 15 (1-3): p. 87-94.