Mycoplasma Contamination – Early Detection and Elimination Can Ensure Research Validity
How big of a problem is mycoplasma contamination? The answer is very big. A variety of studies conducted worldwide have found that mycoplasma contamination in laboratory cultures varies from 15-80% (1). Mycoplasma contamination is a very real concern for research labs, largely due to the fact that contamination can impact cells’ behavior and thus compromise the validity of experimental results and study data. In fact, this widely recognized problem has led many research publications to require authors prove that the cells used in their studies are free of mycoplasma contamination.
After seeing the magnitude of the mycoplasma contamination issue, I was particularly interested in looking at whether contaminated cultures could be saved. I was able to talk with Dr. Shanghao Li, MP Biomedicals about how to help labs deal with mycoplasma contamination, specifically the use of mycoplasma detection kits and removal agents.
Why is mycoplasma contamination so widespread?
There are several factors that contribute to the pervasiveness of mycoplasma contamination. First, it is difficult to detect. Contamination causes no visible signs in the culture and the mycoplasma does not kill the cells. Second, many labs either don’t test at all or don’t test regularly enough to detect and eliminate contamination before it spreads to other lab cultures. Third, cell banks can be contaminated and without proper quality controls, contaminated cells may be introduced into a lab, resulting in the contamination of other cell cultures. Last, there are many sources of contamination, lab personnel, animal sourced raw materials, and the cells themselves. A lack of stringent good laboratory practices can inadvertently cross contaminate cultures in a lab or introduce new contamination.
How to address mycoplasma contamination in research labs?
It is important to create a good quality control system and reinforce good laboratory practices. A key factor in a good quality control system is testing and quarantining new cell lines and instituting regular mycoplasma screening on all existing cell cultures. If you can identify a contamination early then you can prevent it from spreading to other cultures. Early detection also prevents wasting precious resources on a study that is compromised by contaminated cells.
Mycoplasma Contamination Removal
Dr. Li explained that it is critical to detect the presence of mycoplasma in a cell culture early. Once contamination is detected, it is important to remove the mycoplasma without compromising cell viability, thus preserving research validity and rescuing the current study. MP Biomedicals offers a mycoplasma detection kit and Mycoplasma Removal Agent (MRA) to provide a workflow solution to manage both the screening for and the elimination of mycoplasma.
The kit, designed using the Hoechst method, uses the Hoechst fluorescent stain method cited by the Tissue Culture Association (TCA procedure no.75361). It specifically and selectively binds to minor grooves of DNA. It offers in situ detection of mycoplasma and other prokaryotic organisms in less than 2 hours. The kit offers a complete solution and contains all necessary components including stain, diluent, mounting medium and controls.
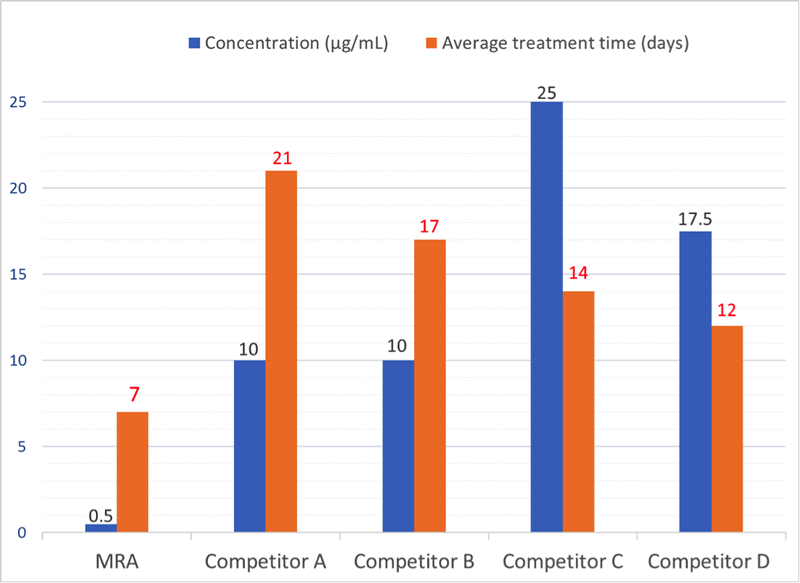
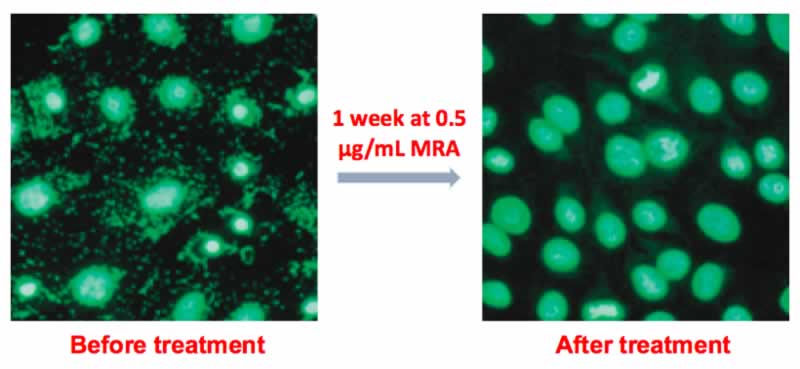
If a mycoplasma contamination is detected, the cells can be treated in culture with the Mycoplasma Removal Agent (MRA). MRA ensures high elimination efficiency and optimal cell viability at the lowest dose for the shortest treatment time (Fig. 1). Only 0.5 µg/mL for one week can keep cell culture mycoplasma free (Fig. 2), as referenced in 550+ leading publications.
I was very interested in this topic and was fortunate to be able to talk with Dr. Li, further about these issues and to get a supplier’s perspective. Our interview is below:
Interview with Dr. Shanghao Li, MP Biomedicals:
How pervasive is mycoplasma contamination in research lab settings?
Mycoplasma is widespread problem with one study estimating 15-80% of research labs worldwide have mycoplasma contamination. Some of our customers have very serious contamination issues and once they do have one contamination, they often have recurring contamination.
By employing regular testing and either discarding or rescuing contaminated cultures with a removal agent, labs can keep mycoplasma contamination from becoming an issue. The first step is to test with a detection kit to see if your culture is contaminated and if it is, you must decide whether to discard the culture or use a removal agent to eliminate the mycoplasma from the culture. Many times labs will choose to discard the culture. However in some situations, it is critical that the culture be rescued and discarding doesn’t make sense. For instance where rare cell lines are being used or if there are no other samples available. Of course if there is significant time and expense invested in the culture, it makes sense to try and preserve the work. In these cases, we recommend that our customers use our Mycoplasma Removal Agent (MRA). We’ve been able to help over 650 customers in the last year to successfully eliminate mycoplasma from their culture and save their research. Our MRA has very limited cytotoxicity and only a low dosage is required, so it is able to eliminate the mycoplasma without destroying the cells.
Why is mycoplasma such a big problem?
It is a big problem for a few reasons. First, it is very difficult to detect. It is very small and can’t be seen even under microscopy. Second, it usually doesn’t present any obvious problems in culture at early stage, so you won’t be able to detect contamination, simply by examining the culture. Third, it is very hard to eliminate it completely from the lab.
Studies done on mycoplasma contamination have shown that several labs don’t test for mycoplasma contamination or don’t test frequently enough. Why do you think labs fail to screen for mycoplasma more regularly?
Since there isn’t any overt negative effect on the culture, many researchers think why bother, especially since in the past testing had to be outsourced and it was expensive and time consuming. However, while there may not be any visible effect on the culture, no overtaking or killing of cells for example, mycoplasma contamination does affect the cells behavior and thus can result in misleading experimental results. It is tragic when years of research are deemed unreliable due to the presence of mycoplasma in the culture.
How does your mycoplasma removal agent work?
MRA is a derivative of the quinoline family of antibiotics, and it eliminates mycoplasma by interfering with DNA replication. The active ingredient in MRA inhibits the mycoplasma DNA gyrase or the topoisomerase IV enzyme, thereby blocking DNA replication and transcription.
How do you see the issue of mycoplasma contamination evolving in the future?
It is good that journal publications and academic thought leaders are recognizing that mycoplasma contamination is a huge issue. With this they are raising awareness that regular screening, detection and elimination is a critical part of the successful functioning of a research lab. Having publications require authors prove that their cells are mycoplasma free has also set a strong inducement for testing cells. Now that there is a way to eliminate mycoplasma, research projects can be rescued not discarded.