New Flexible Bispecific Antibody Format Demonstrates Improved Therapeutic Properties and Manufacturability
This year’s Next Generation Protein Therapeutics & Bioconjugates™ Summit held in San Francisco provided an in-depth look at the latest developments in next-generation protein therapeutic discovery and manufacturing. One talk that I found of particular interest was, “WuXiBody,™ an Innovative and Versatile Bispecific Antibody Format, Opens a New Era For Therapeutic Antibody Development,” presented by George Wang, Ph.D., MBA, Vice President of Biologics Discovery, WuXi Biologics. The talk provided an excellent overview of the promise of bispecific antibodies as well as the associated challenges. Dr. Wang also shared an innovative bispecific antibody platform designed to address many current platform shortcomings.
Bispecific Antibodies
Bispecific antibodies are a very promising next-generation protein therapeutic where different binding regions from different and distinct monoclonal antibodies can be placed on a newly engineered antibody (thus allowing binding to two separate antigens or epitopes) to increase the therapeutic impact. While the development of bispecifics is growing, there are challenges with many of the new platforms. Many current platforms have difficulties with antibody yield coming out of cell culture or after purification, purity, stability, solubility, half-life, and immunogenicity. Any of these issues individually, but especially together, can cause problems with the bispecific’s therapeutic properties and manufacturing feasibility, eventually delaying or blocking delivery of the bispecific to patients. In order to address these issues, WuXi Biologics created WuXiBody™ a proprietary antibody platform.
WuXiBody™ Bispecific Antibody Technology Platform
In the talk, Dr. Wang began with an introduction of the therapeutic value of bispecific antibodies. He explained that there are currently three bispecific antibodies on the market and many in clinical trials. However, bispecific antibodies have been difficult to develop because of their unique biology and complex structure.
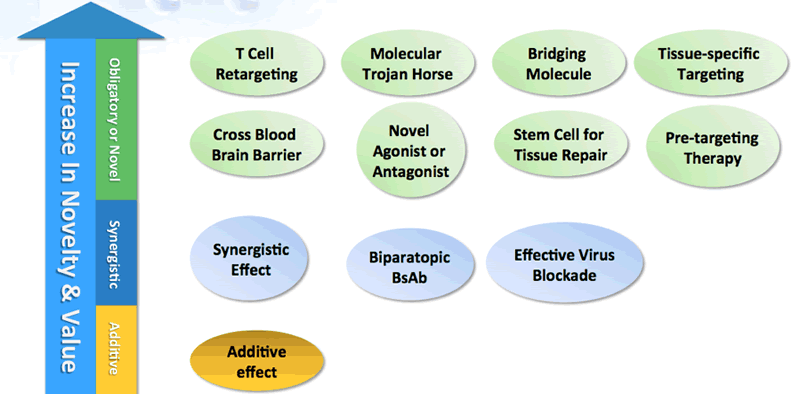
What makes bispecifics so attractive is that there are several different therapeutic strategies and mechanism of action options possible. Dr. Wang presented this chart (Figure 1), which provides an excellent visual of the opportunities that bispecific antibodies present as they progress from the simplest additive effect to more complex mechanisms. As they become more complex, they increase in novelty and therapeutic value but potentially more challenging to develop (Figure 1).
One of the most significant difficulties in developing bispecifics is finding the best format for the molecule. Particularly demanding is finding a combination that provides the upmost safety, pharmacokinetic (PK), pharmacodynamic (PD), physiochemical and immunogenicity profiles. Of course from a practical standpoint, manufacturability is also critical because ultimately the product must be able to be produced at a scale and cost that will make it accessible for patients. Dr. Wang explained that these critical components were all carefully considered when developing the WuXiBody™ bispecific platform.
Dr. Wang then described WuXiBody™ as a flexible bispecific platform that provides various formats to meet a wide range of biological needs. He explained that every bispecific format has unique pros and cons and that there isn’t a single format that can address the needs of all biologics. This is because selecting the best format is dependent on the target biology. WuXiBody™ solves this problem by providing several options within the same flexible format platform. Dr. Wang provided different examples of how WuXiBody™ could be used to meet various biological needs (Figure 2).
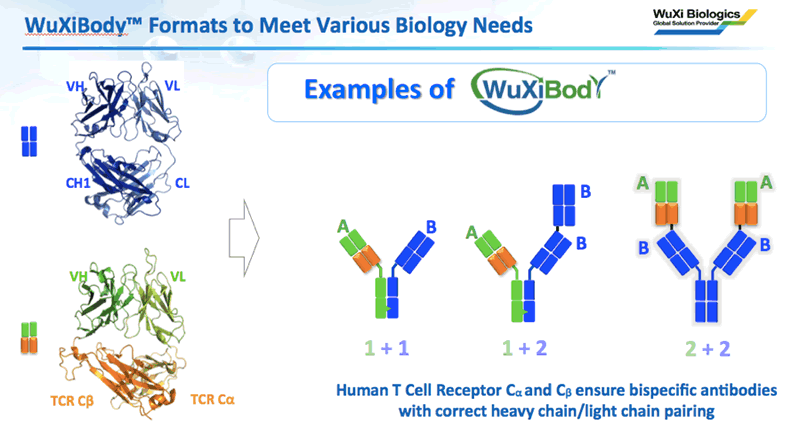
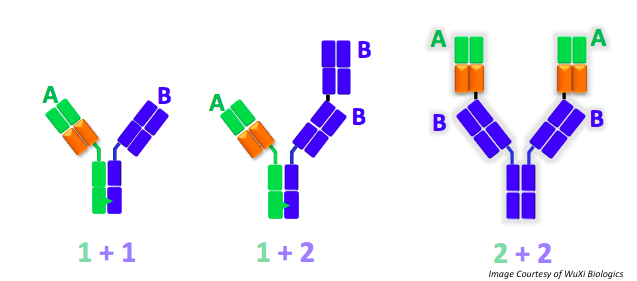
He explained that the flexibility of WuXiBody™ empowers clients to develop novel bispecific antibodies better and faster. WuXiBody™ allows complete flexibility by enabling 1+1, 1+2, or 2+2 binding sites. It also permits almost any monoclonal antibody pair to be easily joined to build a bispecific antibody. Dr. Wang emphasized, “you don’t need a common light chain antibody or a single domain antibody to make a bi-specific with WuXiBody™, any two regular IgG monoclonal antibodies can be easily assembled into a bispecific.”
Dr. Wang then presented data to demonstrate several key benefits of the WuXiBody™ platform, including:
- High yield of greater than 50%
- High solubility with 100 mg/ml
- Good stability at 37° C in serum for over two weeks
- Increased in vivo half-life
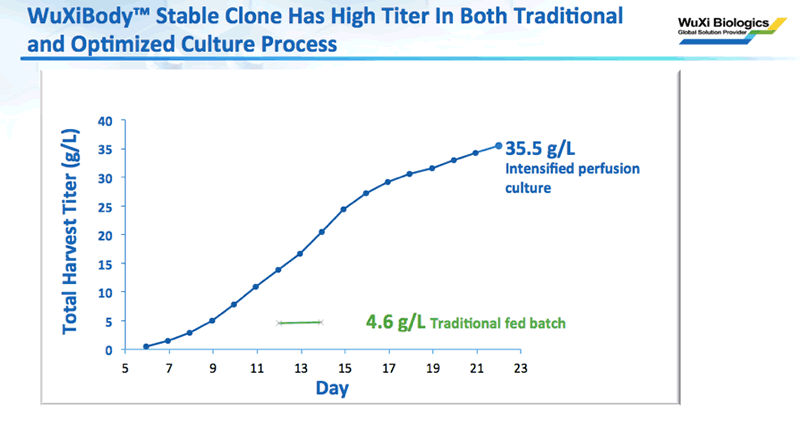
Next, he presented data to show that once a stable cell line was developed, they were able to achieve 4.6 g/L titer in traditional fed batch and 35.5 g/L in intensified perfusion culture (Figure 3).
Dr. Wang went on to clarify that they don’t see much aggregation and even with the high titers they achieve more than 50% purified bispecific yield. His team also measured thermal stability by DSF and DSC and found that they could achieve consistent Tm greater than 57° C. Lastly, they conducted an LC-MS test to ensure correct assembly.
He went on to speak about development timelines for the platform which was also very impressive. Dr. Wang stated that using WuXiBody™ takes approximately 2-4 months to engineer a bispecific after receiving the monoclonal antibody sequences. After that, the development and manufacturing timeline from this new bispecific to IND is only 16-18 months.
WuXiBody™ Case Study
Dr. Wang discussed some of the limitations of current bispecific formats including poor half-life in vivo. He explained that in the case of the FDA-approved bispecific antibody against CD3 and CD19, for example, the short half-life means that patients have to receive their continuous IV infusion of the drug over many days, which is inconvenient for patients. A longer half-life for bispecifics would be desirable. Furthermore, other issues encountered in the production of bispecifics including low yield, purity, stability, and solubility could also benefit from a format improvement.
WuXi Biologics set out to create a bispecific antibody format platform that addressed these issues as well. The following case study provides a test to see if WuXiBody™ would result in a better bispecific antibody. Thus WuXiBody™ 3438 against CD3 and CD19 was developed and tested for yield, purity, stability, specific binding to target cells, T cell-directed killing of tumor cells in vitro, demonstrated in vivo efficacy and long half-life.
Using an asymmetric WuXiBody™ bivalent 1+1 format they created WuXiBody™ 3438. They first looked at the benchmark antibody and found that expression was quite low with yields of 6 mg/L and a very difficult purification process. The benchmark antibody required a three-step process with only 96% purity. In contrast, WuXiBody™ 3438 reached 115 mg/L yield and 99% purity with a two-step purification. Stability was also a key test and with WuXiBody™ 3438 they reached serum stability at 37° C for 14 days.
Next, they tested the ability of WuXiBody™ 3438 to bind to CD19 + cells and CD3+ cells. They could see the dose-dependent binding on human T cells and B cells.
Additional in vitro and in vivo studies demonstrated that WuXiBody™ 3438:
- Binds to the T cell and B cell targets separately and simultaneously.
- Effectively directs T cells to kill CD19+ cells.
- Has tumor cell-dependent T-cell activation: in absence of target cells, WuXiBody™ 3438 does not induce the expression of T cell activation marker CD25 or CD69, nor stimulate cytokine release of T cells.
- Can kill tumor cells at much lower concentration than induces cytokine release.
- Inhibits CD19+ Raji tumor growth in vivo in a dose-dependent manner.
- Exhibits significantly longer half-life than the benchmark antibody’s 1-6 hours.
In summary, Dr. Wang pointed out that WuXiBody™ is a versatile bispecific platform with the flexibility to fit any target biology. Using the case study, they demonstrated that anti-CD3 and CD19 WuXibody™3438 is a potent and safe bispecific antibody with improved manufacturability and pharmacokinetics. He closed by saying that the WuXiBody™ platform has already been licensed to multiple biotech companies.
We were fortunate to be able to interview Dr. Wang after his talk. Here is a transcript of our interview.
Questions
In your symmetric format do you see any steric hindrance for binding?
Generally, we don’t see steric hindrance in 1+1 WuXiBody™ format. In 1+2 or 2+2 formats, steric hindrance may or may not occur depending on the parental antibodies and the linkers that are used.
What kind of linkers did you use?
We have used several linkers with various sequences and length. One of the linkers is GS linker.
In the asymmetric format did you use your own interpolations or did you use one that is publicly available.
Usually, we use the knobs-into-holes technology that is available in the public domain.
In your talk, you discussed the move from 1+1 bispecifics to more complex bispecific therapeutics. In terms of the more complex therapeutics what do you see as the most promising of the more complex bispecific antibody formats?
The complexity of bispecific antibody is biology-driven and requested by our clients. Generally, our clients asked us to develop more complex bispecific antibodies when there is clear biological mechanism supported by experimental results. WuXi Biologics can efficiently make these complex bispecifics and quickly deliver to our clients.
You presented very impressive timelines when using WuXiBody™, can you share with our readers what is the average development time for bispecifics and how this compares with WuXiBody™?
Prior to developing WuXiBody™, we spoke with many bispecific developers around the world and we even worked on several bispecific antibody projects for our clients and in most cases the development timelines were 24 to 36 months. There were so many obstacles that had to be overcome to have a therapeutic that could be manufactured at scale and also have the safety and efficacy profiles ideal for clinical trials. Now we can typically develop in timeframes similar to monoclonal development. This is a huge advantage for the industry to explore bispecifics now that the development timelines have been reduced drastically.
Your timeline from development to IND is quite fast, can you explain what support you provide to clients in terms of IND filing?
Since we performed all the discovery and engineering and CMC development work there is an inherent efficiency to this single-source development approach. We have all the collective knowledge to share amongst our other functional departments to quickly move from one product development stage to the next and share that information will all relevant technical groups. Because we have a platform for development we have streamlined the technical transfer to large scale GMP manufacturing of Drug Substance and Drug Product as well. We also have a large CMC Dossier writing group that can help expedite the path to IND even further. So not only did we engineer the WuXiBody™ platform to allow faster development timelines by removing the inherent molecular and protein engineering format issues but our single-source CMC development capabilities and extensive capacities also streamline the development path even further.