
Proving Clonality – A Documented Clonality Report for Regulatory Submission
As part of our ongoing poster coverage, we will be writing about some of the posters presented at various conferences. Solentim recently presented a poster at Cell Culture Egineering XV that I thought provided a great solution for documenting clonality to the regulatory agencies. The poster entitled “Proof that can travel – Documented Clonality Report for Regulatory Submission” discusses Solentim’s solution to documenting clonality for regulatory submissions in the form of an easy, rapid report
Background
Clonality is a key factor in cell line development activities and documenting clonality is critical in regulatory submissions. In fact, proving clonality of the Master Cell Bank (MCB) is required in Biological License Application (BLA) submissions. Companies must provide regulatory bodies with proof of clonality or be subject to additional requirements on manufacturing and quality.
Selecting clones/ensuring clonally-derived cell lines can be a manual and highly subjective process that is time consuming and fraught with inconsistencies. These challenges prompted the development of automated cell imaging systems that provide an automated solution for clone selection and images that could provide evidence of clonality.
Cell imaging systems have been evolving and improving over the years to meet increasing imaging demands. Improvements have included, better imaging that permits identification of single cell clones, the ability to see a single cell on the day of seeding and better visibility along well edges.
Another big improvement required however, as this poster points out, is the need for an automated data report generation. Currently users are required to generate their report by cutting and pasting to compile these images into a comprehensive report that could be presented to regulatory agencies but this has been deemed “laborious, error-prone and difficult to audit.”
An Intuitive, Quick to Generate Clonality Report Solution
In this poster, Solentim outlines how the Cell MetricTM CLD can be utilized throughout the Cell Line development process for various applications; monitoring cell growth and verifying clonality (Figure 1) but more specifically how a standardized report to prove the cell line clonality can be automatically generated easily and quickly.
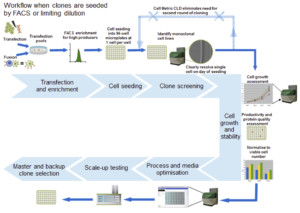
Figure 1: Overview for a typical cell line development workflow with the use of the Solentim Cell Metric CLD for verification of clonality and colony outgrowth measurements.
The method by which the report is generated is quite simple; the plates containing the single cells are imaged at set time points including the day of seeding, to capture a clear image of the cell before it divides, and then periodically over the clones’ outgrowth period. The accompanying software is then able to collate all the plate data for user review. The user can then review each well that shows cell growth and track back to previous well time points (day of seeding) to determine if the clone began as a single cell or not. After the wells are marked as clonal by the user, a report can be generated containing the wells and time points of interest together with any annotation of the well (highlighting the cell of interest or well debris) and any user written comments for individual features in the images. The software then exports this information as a multi-page single PDF report or Powerpoint. This can then be included as part of an IND submission or sent to an internal client e.g. cell banking, or external client in the case of a CMDO.

Figure 2: Example of the PDF version of the Clonality Report generated by the Solentim Cell Metric CLD software.
In addition to the benefit of clone selection that the Cell Metric CLD provides, the new clonality report functionality now also provides the user with the additional benefits of being simple and fast to generate, provides consistency between users, and electronic proof that can easily be presented to regulators e.g. FDA or other stakeholders
For more details and to see the data discussed here, please click on the poster below to view in full size.
Solentim will be attending the Cell Line Development & Engineering Conference, 13th-15th June, at the Parc 55 Hotel in San Francisco, CA. Solentim will be exhibiting at booth #21 and global sales specialists will be available to meet with existing customers and to answer questions.
