Platform Approach to Accelerating Cell Line and Process Development
Cell line development and process development are critical building blocks for successful biomanufacturing. For cell line development, high performing clones must be identified as quickly and efficiently as possible. However, the reality is that traditionally this process has been labor intensive and time consuming.
The clone selection process involves looking for a small number of clones that are expressing sufficient titer of the recombinant protein to become a candidate for production scale. To find these high performing clones often requires screening of thousands of candidates, either manually or through automation. After the high quality clones are selected, process development begins. Process optimization for these clones and specific application is conducted, which can also be time consuming and labor intensive.
As a result of the desire for speed to market, companies are looking for ways to simplify and speed cell line development and process development. For instance, ensuring that more there are more high producing clones. Additionally, optimizing a production platform and selecting clones that perform well in this platform is more efficient than building a process around each clone.
These areas are explored in a recent white paper, “Accelerating Cell Line and Process Development”. The white paper discusses ways to improve the efficiency of cell line and process development by employing a platform approach, which includes all materials needed to advance from protein sequence to production cell line.
CHOZN® and UCOE® Combined Platform to Accelerate Cell Line Development
CHOZN® GS-/- Cell Line
The first component of the platform is the CHOZN® GS-/- cell line. Introduced in 2011, it has both alleles of the glutamine synthetase gene deleted. To generate a cell line producing the gene of interest (GOI), the CHOZN® line is transfected with a plasmid containing the glutamine synthetase gene and the GOI. Absence of glutamine in the culture medium creates selective pressure on the cells, which have integrated the plasmid, allowing them to survive.
UCOE® Technology
The second component is an expression technology that, when combined with the CHOZN® cell line, increases the number of high-performing clones available to select from.
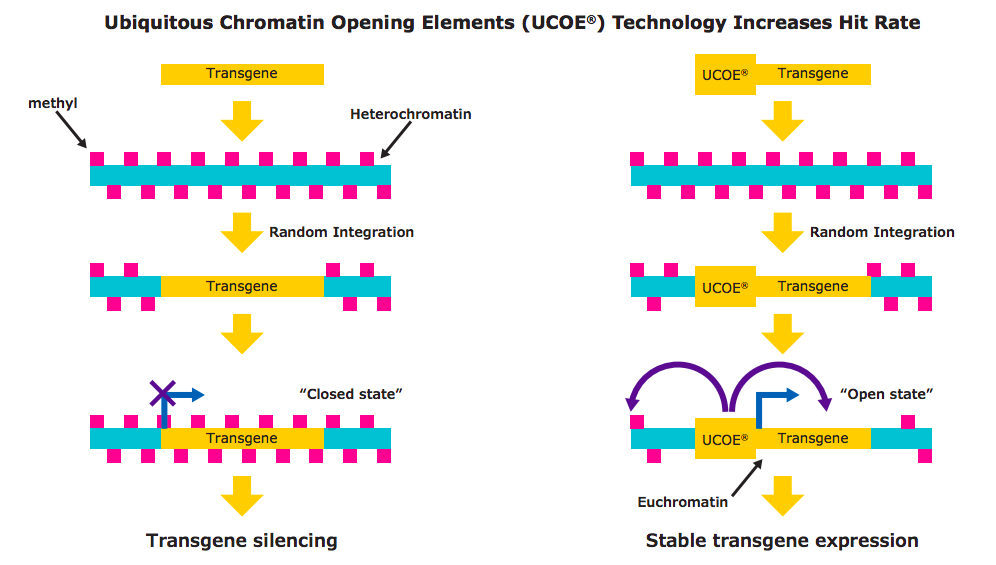
Cell line development relies on random integration of the expression vector into the correct location of the host cell genome in order to permit survival during the selection process. The specific location of the genome into which the transgene integrates, and whether that area consists of heterochromatin or euchromatin, plays an important role in expression of the transgene. Most of the genome is organized into tightly wound, transcriptionally inactive heterochromatin. In contrast, a portion of the DNA is in the open, transcriptionally active form called euchromatin. During random integration, if the transgene integrates into the heterochromatin, it becomes rapidly methylated by host cell enzymes. Following methylation, the DNA is tightly wound into histones and is not capable of supporting transcription.
A UCOE® element is a segment of DNA that derives from the 5′ control region of the promoters of essential housekeeping genes. This region has evolved to prevent the silencing of linked genes. When the transgene is linked to a UCOE® , methylation is prevented and the DNA remains open and capable of transcription (Figure 1). This approach to increase hit rates during cell line development has been used for more than 15 years and has been incorporated into the expression vector included in the CHOZN® & UCOE® combined platform.
Platform Performance
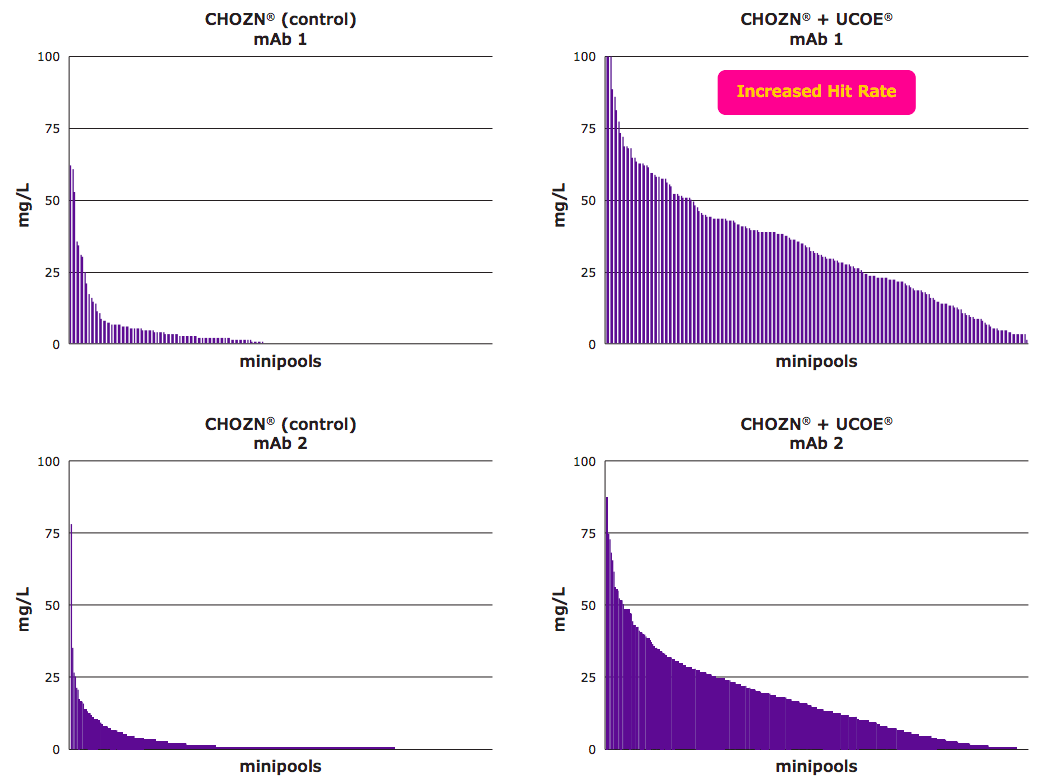
In the white paper, authors study the ability of the CHOZN® & UCOE® combined platform to increase the number of high-expressing clones. In the study, cells were transfected and following a short recovery period, thousands were plated into 96-well plates and cultured in the absence of glutamine for selection. The resulting minipools were then screened for titer. Figure 2 shows the initial screening titers for the UCOE® control minipools in two cell line development studies. Each line represents the titer of a separate minipool, evaluated in a seven-day batch assay on 96 well plates. There was a striking difference between the average titers of the minipools.
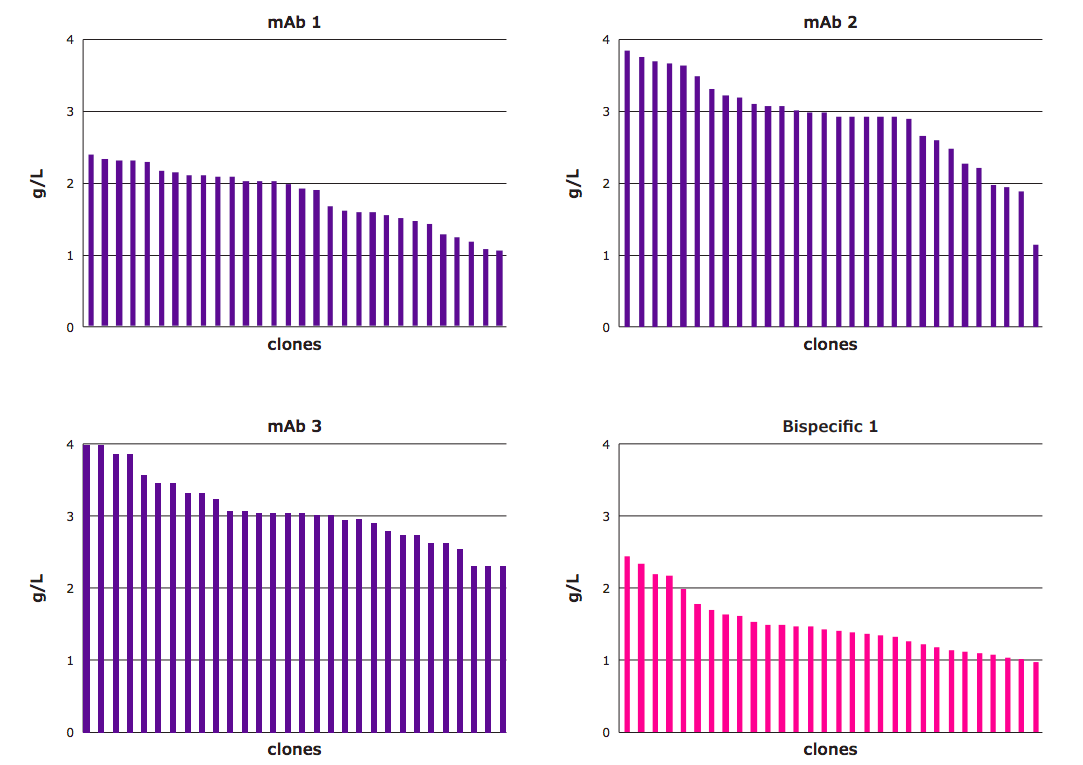
With the increase in the hit rate, study authors hypothesized that it would be easier to isolate high-expressing clones. The top producing UCOE® minipools were seeded for single-cell cloning and those top performing clones were subjected to fed-batch assays. Titers for the top thirty clones from three mAbs and one bispecific cell-line development projects are depicted in Figure 3. These results demonstrated that the CHOZN® & UCOE® combined platform could be used to generate high-performance clones.
Stability
To ensure stability of the top candidate clones, they were exposed to an expression-stability test. Recombinant clones were passaged for at least 45 days; early-passage and late-passage cells were subjected to a fed-batch assay to determine the percentage of retained titer.
Results showed that each of the five clones of mAb #1 retained at least 75 percent titer after 90 days in culture. Seventeen expressing clones from mAb #3 were subjected to a 45-day expression-stability experiment; 13 of these clones maintained at least 75 percent of the early-passage expression. This supported the hypothesis that recombinant CHOZN® & UCOE® combined platform clones exhibit expression stability.
Accelerating Process Development
Another important consideration is how to speed process development. Traditionally, a process is optimized around specific high producing clone. One way to improve upon this is to develop a robust platform process and then only conduct small simple optimizations as needed. In the study, a simple feed screen was conducted with the high-producing clones. The process was then scaled up in Mobius bioreactors to rapidly move process development from bench to pilot scale.
Feed Optimization
For the feed optimization study, clones were evaluated using a simple feed screen study of EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed COMP. Fed batch cultures were grown in spin tubes using EX-CELL® Advanced CHO fed-batch medium. Data from preliminary studies suggested that a combination of the two feeds improved process performance. To evaluate the combination feed, an extra set of tubes for each clone was run, wherein each feed was added in equal parts using the regimen developed for the EX-CELL® Advanced Feed platform. Glucose was added as needed, based on consumption, with the target concentration increased as the cell density increased.
As shown in Figure 5, the different feeds led to differences in cell density and titer for the three different clones; duplicate cultures are represented by the same color, with one solid line and one dashed line.
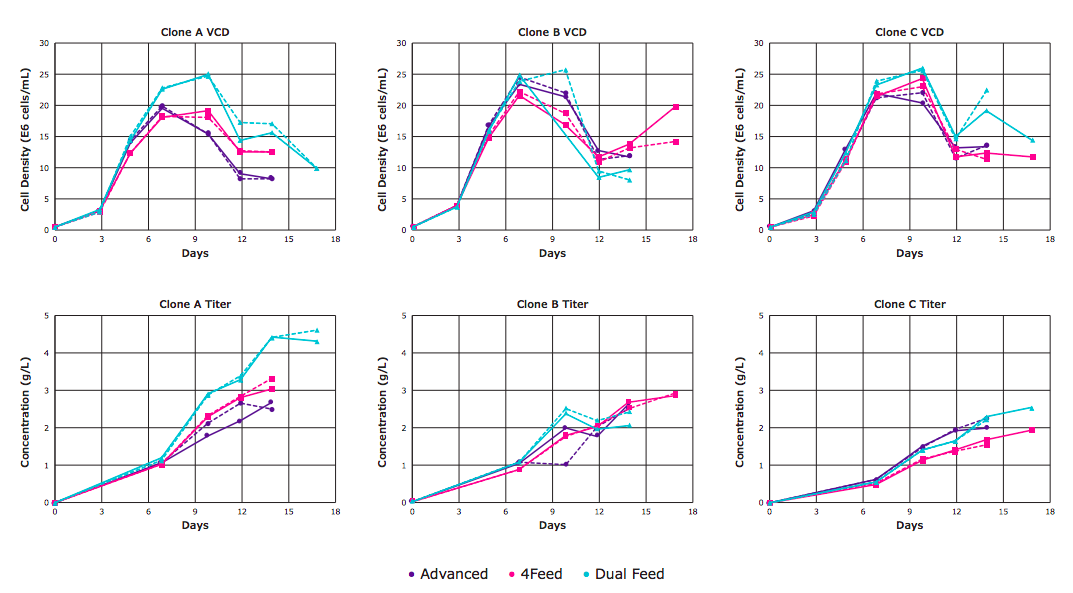
Authors found that the dual feed was beneficial in some of the clones tested, but adding two addition steps at each feed complicated the platform process. Fortunately, these two particular feeds could be combined and fed as a single-unit operation.
Next authors investigated using blend ratios other than 50-50 for the feeds. They found that the preferred feed or blend varied between clones (please see full data in the white paper). Interestingly, the mechanism by which the performance improved varied. In some cases, the preferred feed resulted in an increase in biomass, which subsequently translates to an increase in volumetric titer. While in other cases, there was no discernible increase in cell density, but a significant increase in volumetric titer was observed, indicating increase in cell-specific productivity.
A similar study was also conducted with a more difficult-to-express bispecific molecule produced in the CHOZN® cell line using UCOE® . Similar optimization results were found in this case as well, with full data available in the white paper.
Bioreactor Optimization
Next authors looked at moving the process to bioreactors. A robust and scalable platform process makes this move more efficient and with a higher likelihood of success. As authors state, the foundation of this concept is that it is simpler and faster to pick the clone that fits a robust process than to redesign the entire process for a specific clone for every project. By selecting a clone that fits the process, the bioreactor development timeline can be reduced by months. Instead of designing a process from scratch, minor adjustments can be made, if needed, to the existing process to reach the desired performance level.
In order for a platform process to be successful, process parameters should be set so they are translatable across scales, which allows rapid scaling from bench to pilot and reduces possible process development bottlenecks. This approach enables more rapid technology transfer and generation of material to support toxicology studies or phase one clinical trials. The decision about investing further time and effort on optimization can then be decided based on project phase and commercialization requirements.
Study authors implemented their platform process in bioreactor studies for the selected clones. Clones were first evaluated at 3-liter scale for cell density and volumetric productivity. After the top clone was selected, it was evaluated at 50-liter scale. Comparable results were found between the 3-liter and 50-liter scale bioreactors. Thus, confirming the robustness and scalability of the platform. A similar bioreactor evaluation was also conducted for more difficult-to-express bispecific.
Study Takeaways
- Use of the CHOZN® & UCOE® platform with feed screens and a robust bioreactor scale-up approach delivered a consistent and robust solution for upstream bioprocessing needs.
- The CHOZN® & UCOE® platform increased the hit rate of high-producing clones.
- Implementing a feed-screen step in the development strategy can significantly improve the performance of fed-batch processes; many cell lines and clones have demonstrated increased cell densities and volumetric titer with the use of feed blends. However, it is important to determine what is best for a particular clone as some prefer single feeds and other prefer blends.
- Use of a series of scalable bioreactors, such as Mobius® bioreactors, will significantly reduce process development timelines.
- Development timelines can be significantly reduced by leveraging a robust platform process, which allows for rapid evaluation of top clones in bioreactors and rapid scale up from bench to pilot scale. It should be noted, however, that this approach prioritizes speed over perfection. It can help get a process ready for tech transfer sooner, which translates into generating material for toxicology studies and phase-one trials earlier. However, there are aspects that can be optimized to improve process performance further, which is important when locking in a process for phase two and beyond.
To see the full study results, please see – Accelerating Cell Line and Process Development