Polyplus FectoVIR®-AAV GMP Enables Large Scale AAV Transient Transfection
A highly effective transient transfection reagent that can be used to produce high-quality, regulatory compliant therapeutic viral vectors for patient administration.
The field of cell and gene therapy is expanding rapidly, which has increased the demand for the viral vectors used for in vivo and ex vivo gene transfer. Adeno-associated viral vectors (AVV) are the preferred vectors thanks to their safety profile, broad tropism, and non-integrating properties. In the quest to achieve the high AAV quantities necessary to treat patients, there is a need for industrialized scalable viral vector production platforms that adhere to regulatory good manufacturing practice (GMP) guidelines.
Transient transfection of host cell lines, predominantly adherent HEK293 cells, is the simplest and fastest approach for large-scale production of therapeutic AAV particles. In efforts to intensify upstream processes and improve AAV productivity, viral vector manufacturers are switching to suspension-adapted HEK293 bioreactor cultures. However, better reagents for large-scale transfection are needed to overcome the efficiency and productivity constraints of current transient transfection platforms.
Polyplus, the maker of PEIPro®, has developed a novel transfection reagent to improve scalability, productivity, and flexibility for industrial manufacturing of AAV viral vectors in suspension cells. FectoVIR®-AAV GMP is an animal free, GMP grade transfection reagent designed for large scale transfection used in commercial manufacturing. FectoVIR-AAV-GMP is manufactured using a fully validated process in compliance with Pharma ICH Q7 and Part II GMP guidelines to ensure traceability and patient safety from starting material to the final product. Regulations dictate that all raw materials, excipients, and ancillary materials used to manufacture therapeutic AAVs should move to a fully GMP compliant supply to ensure patient safety. Using compliant raw materials from the start can be highly advantageous, especially considering that any changes made during late-stage development involves additional regulatory verification that can extend timelines and add extra cost.
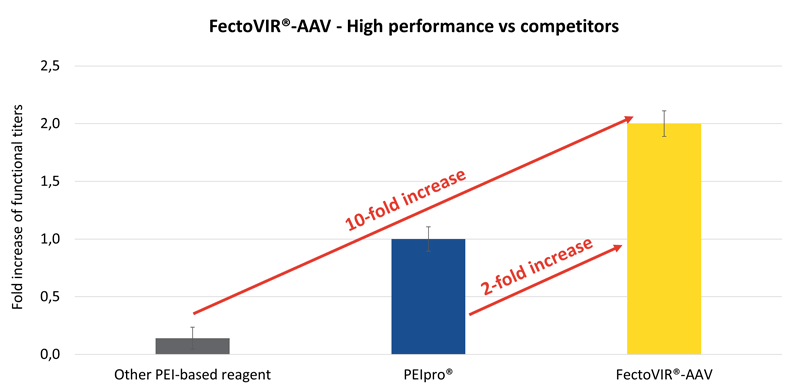
FectoVIR-AAV GMP offers superior AAV productivity in suspension HEK-293 cell systems with up to 10-fold increase in functional titer yields compared to competing transfection reagents (Figure 1). It can support commercial scalability by addressing both time and volume limitations of large-scale transfection. At large scale, the time required for premixing and addition of large complexation volumes to bioreactors can significantly impact viral titer yields. Timely addition of DNA/transfection reagent complexes to suspension cultures is necessary to prevent unwanted aggregation and allow efficient binding to the host cell for endocytosis. FectoVIR-AAV GMP has been optimized to reduce the complexation volume compared to traditional workflows (10%) to half (5%) or even one tenth (1%). As well, the transfection complexes formed using the reagent are stable for up to 6 hours alleviating technical constraints during the preparation and transfer of large complexation volumes into large scale bioreactors.
FectoVIR-AAV GMP is a highly effective transient transfection reagent that can be used to produce high-quality, regulatory compliant therapeutic viral vectors for patient administration, from clinical trials through to commercialization. The reagent offers viral vector manufacturers a commercially viable production platform that can achieve high AAV titer yields. This greatly expands the number of doses per production batch to meet growing industry demand in a robust and cost-effective way.
For more information, please visit: FectoVIR-AAV GMP