
Production of MSC-Derived Exosomes Using Bioreactor Systems and Controllers
Mesenchymal stem cells (MSCs) have been at the epicenter of regenerative medicine since their identification in the 1970s, due to their ability to differentiate into various mesoderm-derived cell types, their immunomodulatory function through paracrine factors (secretome), and ability to home to sites of tissue injury. MSC-based cell therapies are promising for the treatment of a variety of diseases and conditions like wound healing, osteoarthritis, cardiovascular disease, and neurological disorders; however, problems associated with inadequate cell localization and low cell survival rate within the target tissue make the MSC challenging. Because many of the therapeutic effects of MSCs are mediated by the secretion of soluble paracrine factors, researchers are interested in the therapeutic potential of exosomes, which are the central mediators responsible for paracrine functions.
What are MSC-Derived Exosomes?
Exosomes are small nanoscale extracellular vesicles that transfer functional molecules like cytokines, proteins, nucleic acids, and 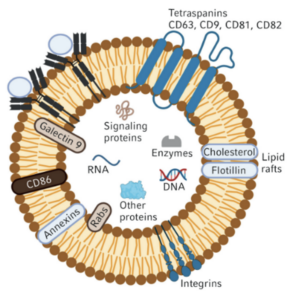 lipids from MSCs (one of the many cell types able to secrete exosomes) to the target cells. They also actively participate in cell-to-cell communication and contribute to the healing of injured or diseased tissues and organs. Exosomes have gained increasing attention as potential cell-free alternatives to MSC-based cell therapy. They offer several compelling advantages compared to cell-based approaches including exceptional stability, biocompatibility and lower immunogenicity. MSC-derived exosomes are smaller and more easily delivered to target tissues than whole cells, and can cross biological barriers, such as the blood-brain barrier.
lipids from MSCs (one of the many cell types able to secrete exosomes) to the target cells. They also actively participate in cell-to-cell communication and contribute to the healing of injured or diseased tissues and organs. Exosomes have gained increasing attention as potential cell-free alternatives to MSC-based cell therapy. They offer several compelling advantages compared to cell-based approaches including exceptional stability, biocompatibility and lower immunogenicity. MSC-derived exosomes are smaller and more easily delivered to target tissues than whole cells, and can cross biological barriers, such as the blood-brain barrier.
While MSC-derived exosomes have shown promising results in preclinical studies for the treatment of a variety of conditions, more research is needed to fully understand the functional mechanisms by which exosomes promote tissue repair and regeneration and there are still unresolved concerns to address before bringing MSC-derived exosomes into the clinical setting. As interest grows, there is a need to upscale MSC culture processes to enhance exosome production and one such approach involves increasing the volume of cell culture from flasks to bioreactors.
Production of MSC-Derived Exosomes Using Single-Use Bioreactors Systems
Eppendorf has conducted a process development study to determine the feasibility of producing MSC-derived exosomes using the BioBLU® 1c Single-Use Bioreactors and SciVario® twin bioreactor control system designed for individual or parallel control of up to two small bench-scale devices. Human induced pluripotent stem cells (hiPSC)-derived MSCs were cultured on collagen-coated microcarriers using the BioBLU single-use bioreactors and parameters such as cell growth, viability and metabolic activity (levels of glucose, ammonia and lactate in the medium) as well as the exosomes abundance were evaluated at different timepoints throughout the culture run.
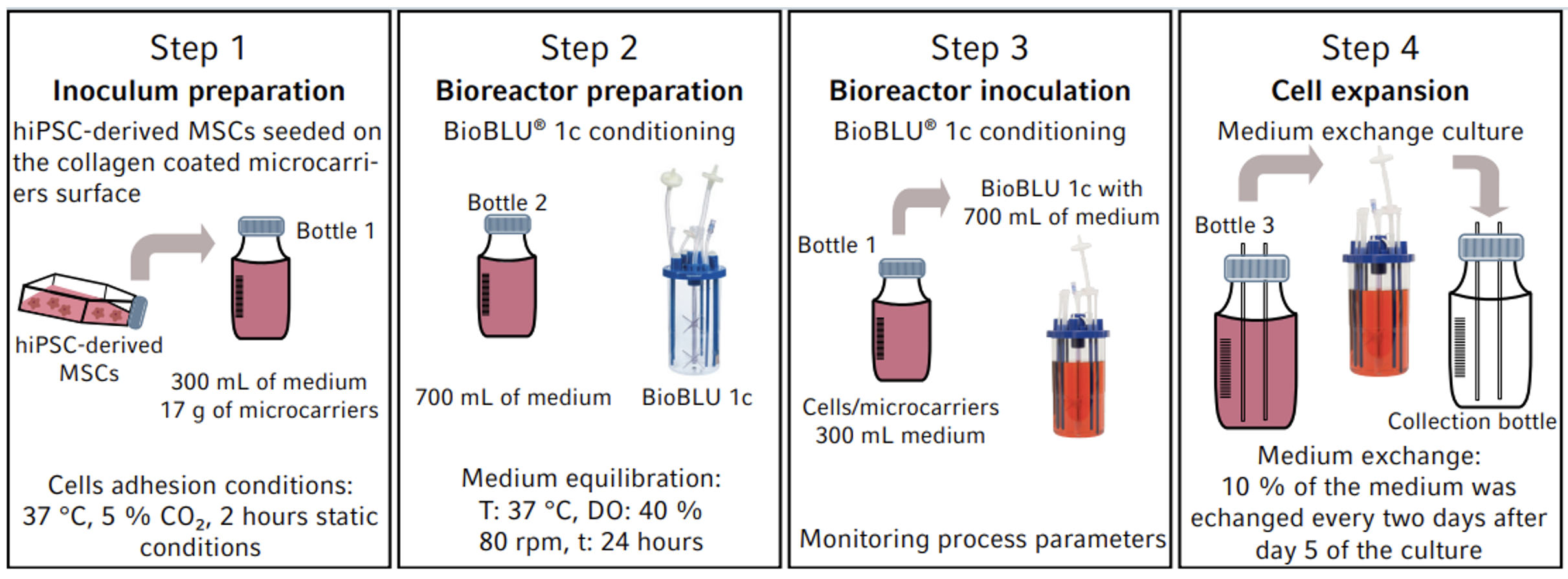
The utilized BioBLU 1c Single-Use Bioreactor is equipped with a single pitched-blade impeller. Each unit has three universal port connectors for pH and polarographic dissolved oxygen (DO) sensor (Mettler Toledo®), a temperature control block that combines electrical heating and water cooling, agitation control, and a gas module that includes 1 TMFC (Thermal Mass Flow Controller) and 4 solenoid valves for automated 4-gas mixing.
The SciVario twin bioreactor controller automatically detected the pH and DO measurements with the integrated digital sensor technology. With a wide range of pumps and a gassing system, the controller was also used to establish a DO cascade to automatically control the oxygen levels based on set parameters as well as to perform medium exchanges every two days for the culture duration after day 5 to support logarithmic growth.
The hiPSC-derived MSCs were immunophenotyped using CD90, CD29, CD34, and CD11b markers at passage 3 to check for stemness prior to transfer into the BioBLU bioreactors. On days 5, 8, 11, and 14 of culture, 50mL of the MSC culture were harvested from the bioreactor and incubated separately for 48hrs to isolate the exosomes. Enrichment and purification of exosomes was done according to the ExoQuick-TC PLUS (System Bioscience, EQPL10TC-1) protocol with some modifications. The abundance of tetraspanin-containing exosomes was quantified using the ExoELISA-ULTRA CD63 Kit (System Bioscience, EXEL-ULTRA-CD63-1).
The results from the study demonstrates the feasibility of producing hiPSC-derived MSC exosomes using Eppendorf SciVario  controller and BioBLU bioreactors. A direct correlation between MSC density and secreted exosomes was observed with a steady increase of exosomes from day 5 to day 16 (from 2.6 x 1010 to 8.6 x 1010). The design of the SciVario system allows the precise control and manipulation of the cell culture environment to support rapid proliferation of the hiPSC-derived MSC cultures. The compact, adaptable design and its intuitive touch user interface make it the ideal controller in R&D and process development. The experiments shown in this application note are preliminary studies and have not yet been optimized to maximize exosome production levels but the data gathered can serve as a guideline for further improvements in MSC-derived exosomes isolation, purification, and scale.
controller and BioBLU bioreactors. A direct correlation between MSC density and secreted exosomes was observed with a steady increase of exosomes from day 5 to day 16 (from 2.6 x 1010 to 8.6 x 1010). The design of the SciVario system allows the precise control and manipulation of the cell culture environment to support rapid proliferation of the hiPSC-derived MSC cultures. The compact, adaptable design and its intuitive touch user interface make it the ideal controller in R&D and process development. The experiments shown in this application note are preliminary studies and have not yet been optimized to maximize exosome production levels but the data gathered can serve as a guideline for further improvements in MSC-derived exosomes isolation, purification, and scale.
For more details on the experimental design, process parameters and more, please download the application note here: Stem Cell Exosome Production on the SciVario® twin,
a Flexible Controller for Your Bioprocess Needs.