Pumping Iron – But Not in the gym: The Critical Roles of Transferrin in Cell Culture Media
Since the early days of cell culture, scientists have typically relied on serum supplementation in their cell culture media, most often in the form of whole bovine or human-derived serum or isolated components/fractions thereof. This ill-defined mixture of proteins, small molecules, and other factors has served as a physiological crutch compensating for our limited understanding of the complex ingredient interactions required for in vitro propagation of mammalian cells. As cell culture-based therapeutics have transitioned from bench research to clinical trials at bed side, they have established the desperate need for a great “cell cultural enlightenment” – and our days of blissful ignorance must come to an end.
Driven by many factors, but in part due to the inconsistent nature of blood derived products, and more so due to safety concerns (e.g. BSE transmission from bovine blood products or Hepatitis/HIV risk from human derived products), there has been a great push toward understanding the delicate art that is cell culture media design, and in doing so, eliminating all serum-derived components in the hope of maximizing performance, reliability, and safety. In this series of blogs we will be highlighting the roles and functions of various ingredients and supplements used in lieu of serum in typical cell culture medias with the hopes of shedding some light into this often overlooked, but ever important topic. For this first blog, we will be discussing the roles and functions of transferrin and its importance in maintaining the cell’s iron homeostasis.
Transferrin
A quick survey of researchers to name the first iron binding protein that comes to mind almost assuredly will result in hemoglobin being chosen – possibly accompanied by flashbacks of general biochemistry and all-nighters studying allosteric oxygen binding. Although it may not get the same attention as hemoglobin, transferrin is just as important, and the functions of this protein in cell culture will be detailed below.
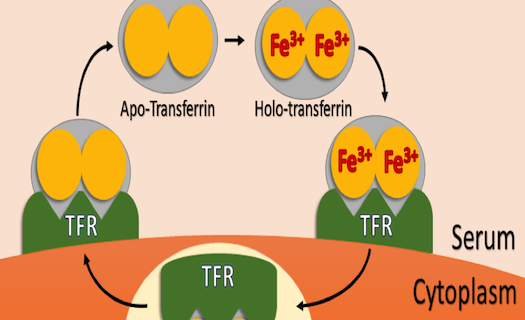
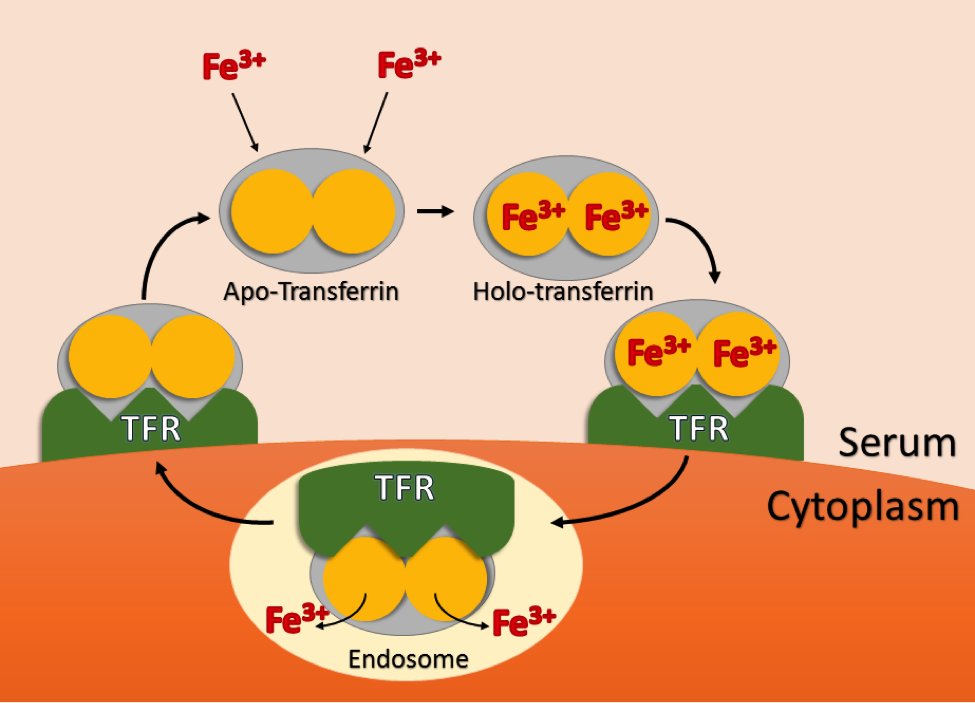
So, what does transferrin actually do? This bi-lobed protein’s primary function is the delivery of iron to cells in situ and in vitro. Although almost all life on earth requires iron to function, its uptake and delivery must be highly regulated. Too little iron can have negative effects on the cell’s ability to survive and proliferate, while too much iron can result in oxidative damage due to the generation of superoxide and free radical species. Transferrin is nature’s solution to balancing iron uptake. Free transferrin in an organism’s blood serum or in media may exist in one of four forms, diferric holo-transferrin, mono-ferric N or mono-ferric C lobed transferrin, and apo-transferrin that has no iron bound. Holo-transferrin has the highest affinity to the cell-surface transferrin receptor (TFR), followed by the mono-ferric forms, with apo-transferrin having the lowest affinity. Upon binding one or two Fe3+ atoms in the serum, transferrin then binds to TFR, and the TFR-transferrin complex is then endocytosed via the clathrin coated pit pathway. Protons are then pumped into the endosome, and the subsequent drop in pH results in release of iron from transferrin. The transferrin-TFR complex is then shuttled back to the cytoplasm, and transferrin is released from the receptor where it can scavenge two more iron atoms from the media or blood. Figure 1 illustrates this cycle in cartoon form.
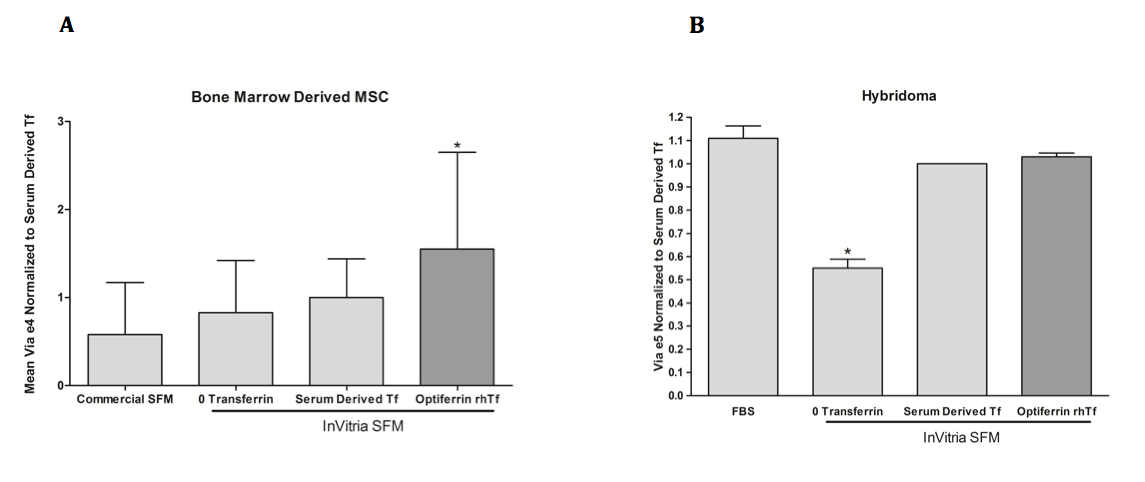
So now we understand the importance of transferrin in cell culture media, but are there any alternatives to this iron delivering protein? Various iron compounds and chelating agents have been investigated for this purpose, however none of these have been able to match the efficacy of transferrin. The complex chemistry of iron in the cell and media necessitate extreme control of its concentration and delivery, and getting the proper balance has proven to be an extremely challenging task. Due to this, supplementing serum free and animal component free media with recombinant transferrin is the best practice for ensuring maximal cell viability and proliferation. InVitria’s animal component free transferrin, Optiferrin, is an excellent option when looking to make the transition to serum free media. Made in the USA, this product is high purity, is regulatory friendly, and will undoubtedly meet and exceed all of your research team’s needs and expectations. Importantly, Optiferrin demonstrates excellent biological activity in numerous cell types. This is demonstrated for MSC and murine hybridoma cells in Figure 2. Please visit our website to learn more about Optiferrin, and how we can help you meet your animal component free cell culture needs.
Please visit InVitria’s website at www.invitria.com to learn more about Optiferrin