Real-time Mycoplasma Contamination Detection for Biomanufacturing
Biomanufacturing contamination due to Mycoplasma is a very real concern, as it poses a potential health risk for patients. As such, regulatory agencies require biopharmaceutical companies to test for the presence of mycoplasma both during the manufacturing process and in the final product. While any contamination can be extremely challenging and costly, mycoplasma contamination is particularly difficult.
The presence of mycoplasma in cell culture has been described as pervasive. According to the publication” The scope of mycoplasma contamination within the biopharmaceutical industry, “contamination rates in established cell cultures have been reported between 15 and 35% with considerably higher occurrence cited in certain selected populations.” Its pervasiveness could be due to several different factors, with one certainly being the fact that mycoplasmas can have a broad range of hosts including humans, animals, insects and plants. The small size of mycoplasmas also makes them problematic to detect and remove via filter. Mycoplasmas thrive in cell culture environments because they find nutrients in medium and can grow to high concentrations without initially causing obvious problems to the host cell or culture productivity.
Release Testing Requirements
As discussed, biopharmaceuticals must be completely free of mycoplasmas and regulatory authorities require release testing as part of the manufacturing process. Traditional methods for mycoplasma testing as described in the USP (U.S. Pharmacopeial Convention) and EP (European Pharmacopoeia) involve the culture method and/or indicator cell culture testing. These testing methods, while well defined and widely accepted, are difficult to administer because they require highly trained personnel to administer and can require up to 28 days for results. In addition, difficult to cultivate or non-cultivable mycoplasma species can result in a mycoplasma contamination going undetected. As a result, many regulatory agencies now also accept rapid nucleic acid amplification techniques (NAT) such as real-time quantitative polymerase chain reaction (qPCR) for mycoplasma testing. In addition to final release testing, qPCR testing for mycoplasma enables a more robust in-process control strategy.
qPCR Solution
PCR testing for mycoplasma has been gaining acceptance for some time and Roche CustomBiotech recently presented a poster detailing the validation of their MycoTOOL Mycoplasma Real-Time PCR Kit (MycoTOOL RT) conducted by Roche Pharma (Penzberg, Germany) based on EP2.6.7 NAT validation guidelines. The United States, European, and Japanese pharmacopeias list nucleic acid tests (NAT) as a mycoplasma testing option provided that they are properly validated.
Poster Highlights
The poster nicely outlines workflow for manual and automated processes and presents data on the validation study results.
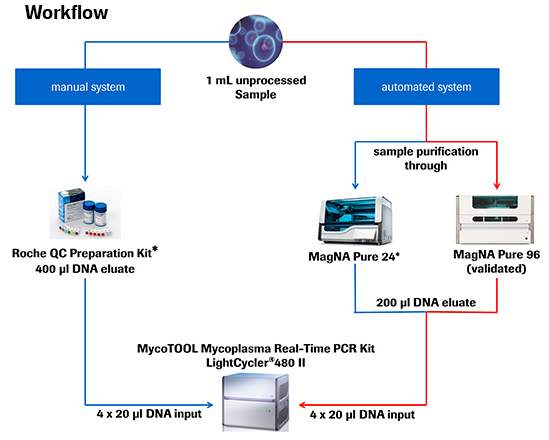
MycoTOOL RT Workflow
The MycoTOOL RT is compatible with either manual or automated protocols and testing can be conducted in less than 5 hours for both (Figure 1). This is a significant advantage that PCR has over more traditional methods.
Another advantage for MycoTOOL RT is that it utilizes highly specific TaqMan® probes, which permit the detection of both cultivable and non-cultivable mycoplasma species. This reduces the risk of a contamination going undetected.
Validation Study Results
The study outlined in the poster below, demonstrates compliance of the MycoTOOL RT with the EP2.6.7 NAT validation guideline, showing that the assay is sensitive, specific, robust, precise and comparable to compendial methods for CHO manufacturing processes.
In the poster, Roche CustomBiotech presents data on limits of detection (LOD), test specificity, test robustness, precision, absence of cross contamination and comparability as part of their validation process. Please refer to the poster for specific details and methods.
A critical part of the data was the results of the comparability study, which concludes that all three of the mycoplasma detection methods: culture method, indicator cell culture method, and MycoTOOL RT were able to detect mycoplasma contaminations with a sensitivity ≤10 CFU/mL. However, MycoTOOL RT was also able to detect strains that are non-cultivable.
Conclusion
It makes sense that biopharmaceutical companies are moving toward a more rapid mycoplasma detection method. With mycoplasma testing that is now quick and efficient, it is a tool that can be more widely used and can serve more purposes. For instance, mycoplasma detection testing could now be used as an inbound quality control step for raw materials or as an in-process early detection approach to avoid further contamination, loss of time, and resources.
For full details, please click poster below:
For more information, please see MycoTOOL RT.
Mycoplasma Photo: © R. Rosengarten, Mycosafe, courtesy of Roche
The MycoTOOL Mycoplasma Real-Time PCR Kit is for use in quality control/ manufacturing processes only. MYCOTOOL is a trademark of Roche. Other brands or product names are trademarks of their respective holders.
