
Scalable Viral Vector Upstream Process for AAV Gene Therapy Manufacturing
Gene therapy is an exciting area of medicine that has the potential to treat a wide range of diseases for which no other treatments are available. One of the key components of successful gene therapy manufacturing is the production of the viral vectors that are used to deliver the gene of interest. Viral vector systems are by far the most widely used methods to deliver therapeutic gene products because of their infectious nature and ability to introduce specific genes into a cell.
However, production of viral vectors, particularly the efficient scale up of manufacturing systems, is one of the biggest challenges that the industry faces today. Généthon, a gene therapy company looking to move into clinical trials with a scalable high-capacity and cost efficient process for viral vector manufacturing, partnered with Pall on a project to assess the iCELLis® single-use, fixed bed bioreactor system for both viral vector production and scalability.
The iCELLis bioreactor is a fully-integrated, high-cell density bioreactor that provides a 3D environment with low shear stress for adherent cells. This design enables high cell densities and high productivity. iCELLis technology is linearly scalable as the fixed-bed height can be kept constant from bench-scale (0.5 to 4 m2 ) to manufacturing-scale (66 to 500 m2 ). The iCELLis system also provides process automation and control via single-use sensors and a fully integrated control system, reduced footprint and capital investment, and ease of process scale-up.
Method of Evaluation
The study evaluated the iCELLis technology for AAV-8 production via transient transfection of HEK-293 cells. Généthon’s process was transferred to the iCELLis Nano bioreactor for evaluation at bench scale. Results showed that the HEK-293 cells adhered to the carrier within 6 hours and presented an exponential cell growth profile. No medium optimization was required to adapt the process to the iCELLis, however medium optimization significantly improved the specific productivity with up to an 8-fold increase. The AAV-8 production process was developed and scaled-up in the iCELLis Nano bioreactor. Final upstream process and main parameters are presented in the flow-chart below. AAV productivity was assessed at 72 hours post transfection (hpt) by qPCR performed directly on cell lysate.
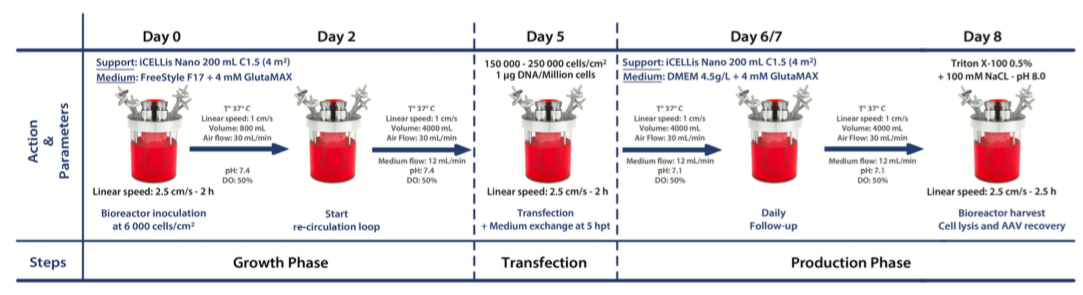
Results
Results of the study were published in a recent poster, “Development of a Scalable Viral Vector Upstream Process for Gene Therapy: rAAV-8 Production by Transient Transfection of HEK-293 Cells in iCELLis® Bioreactor.” In brief, results demonstrated that AAV-8 productivity in the iCELLis Nano bioreactor 4 m2 provided good process scalability and reproducibility. The AAV production process was successfully scaled-up from 2 to 10 cm height while maintaining specific productivity. In addition, the study showed that the pDNA/cell ration could be reduced by 50% thus improving cost efficiency, as pDNA is one of the main cost drivers in viral vector manufacturing.
These results show that iCELLis bioreactors are a promising scalable technology for clinical production of AAV Gene Therapy products. Studies are ongoing to evaluate the quality of the vectors in order to consider a scale up at 500 m2 .
For more information, please see “Development of a Scalable Viral Vector Upstream Process for Gene Therapy: rAAV-8 Production by Transient Transfection of HEK-293 Cells in iCELLis® Bioreactor“
