Selecting the Appropriate Scaling Strategy for Different Cell Therapy Applications
 This article is one in a series that was published in the eBook
This article is one in a series that was published in the eBook“Cell Therapy Production”.
You can download all the articles in the series, by downloading the eBook.
Cell therapies represent a new paradigm in medicine, and the commercialization of several treatments has prompted a need for those developing these therapeutics to look at the scalability of their manufacturing platforms. The decision on how to scale-up and scale-out while choosing the appropriate platform and associated technology relies heavily on the therapeutic being manufactured. Because the cells are the therapeutic product, they need to retain their phenotype and functionality regardless of the manufacturing method. Utilization of primary cells to develop these therapeutics presents unique challenges. These include addressing issues such as donor variability, increased sensitivity to changes in their environment and downstream considerations for formulation and final fill.
In considering the different strategies of both autologous (patient- derived cells) and allogeneic (cells from a donor) cell therapies, it is recognized that a single model for scaling production is not suitable. Allogeneic therapies fit into a more traditional, centralized manufacturing model, where one therapeutic batch can treat many patients. In contrast, autologous therapies necessitate a de-centralized model, where manufacturing occurs near the point of care due to the patient-specific nature of the therapy. Here, one patient equals one manufacturing batch, which limits batch volumes and associated economies of scale at that level.
All said, there are some key considerations that should be examined when looking to scale these therapies for manufacturing. In this article, we aim to provide guidance for scaling-up and scaling-out while considering those factors that are essential to identifying the optimal platform required to manufacture a cell therapy product for clinical use.
Scale Up and Scale Out
When considering strategies to expand the number of cells being grown, considerable effort is focused on the decision to scale up and scale out. By definition, scale-up of a process involves using larger vessels to increase the volume of production. In scale out, the culture vessel remains the same, but more units are added to a system to increase production capacity. To illustrate this, Figure 1 shows an example of scaling-out where a researcher moves from using a single cell culture flask to multiple flasks of the same dimension before scaling-up to a larger format. In this scenario, scaling up would include moving to larger size flasks or other high throughput stacked vessel systems such as the Corning® CellSTACK®, HYPERFlask®, HYPERStack®, or CellCube® for attachment- dependent cells, or spinner and shaker flasks for cells in suspension.
While considerations for scaling out a production process can be completed quickly, because the unit of production remains the same, the considerations for scaling up can often involve more planning. Questions will focus on the time needed to optimize cell culture expansion, harvest and quality testing at the process development stage, through pilot-scale production, and up to large-scale manufacturing. There is a need to invest time at the process development stage to identify potential challenges that would increase difficulty in transferring the process to manufacturing scale. Therefore, it is important to proactively identify and address potential issues during process development that could be barriers to large-scale manufacturing. In cell therapy, where the functionality of the cells is paramount, scaling strategy requires significant validation to ensure cells retain the same phenotype and quality at each stage of the process.
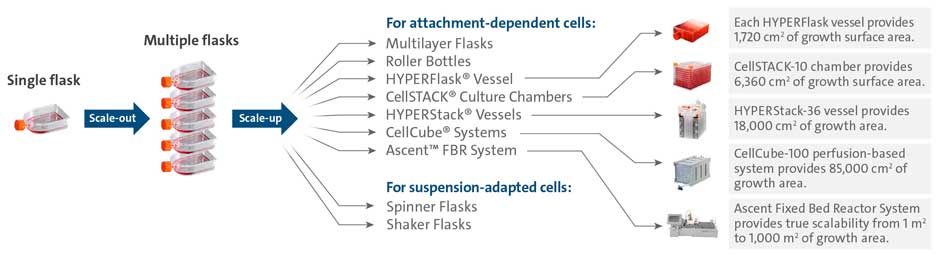
There is certainly a place for both scale-out and scale-up approaches but the decision largely depends on factors like type of cell therapy or biology of the cells, timelines (i.e. time to market, product demand), facility space, availability of skilled technicians and capital investment/budgetary constraints. Timely and effective planning will facilitate a successful transition to increased production capabilities.
Also, depending on product demands or company needs, the decision could be made to switch to a technology different than the one used for development. For example, a developer might decide to switch from adherent, static cell culture to a non-adherent, suspension culture system to meet production demands.
1) Cell Type Considerations
In cell therapy, many of the commercially available adoptive cell therapies target primary cell types, like cytotoxic T cells to generate chimeric antigen receptor (CAR)-T cell therapies. These cells are non-adherent and can be cultured in suspension systems. Scaling up suspension-cultured cells to larger volumes/vessels is a complex operation that requires careful agitation/mixing to ensure sufficient gas and nutrient exchange while minimizing cell damage caused by shear stress. It is important to assess the flow dynamics based on the equipment (i.e., number of impellers, sparge strategies, stir speed, mix strategy) and their impacts on cell type. These issues can present unique challenges, as often times multiple vessel designs are utilized in a scale out process, but identifying and resolving them are critical to maintaining product quality. Additionally, utilization of process analytical technology, commonly used in large-scale protein production, can help with cell therapy manufacturing optimization by providing critical information about the health and behavior of the culture.
As interest grows in allogeneic cell therapies approaches like CAR-T, several biopharmaceutical companies are investing in induced pluripotent stem cells (iPSC) as their T-cell source, which can be expanded and differentiated in vitro to meet the cell number and dosage requirements for cell therapy1. The expansion of iPSCs is commonly performed using two-dimensional adherent conditions in feeder-dependent systems with embryonic fibroblasts or feeder- free systems with purified matrices. Robust large-scale production of these cells is a major challenge since these cells are prone to unwanted, spontaneous differentiation and cell death in suspension culture. Intermediary microcarrier-based systems are a useful culture format for iPSCs to maintain their attachment requirements. Microcarrier-based systems allow for process scale-up to stirred suspension bioreactors compatible with process analytical technology to enable real-time monitoring. However, adapting to a new microcarrier system from static, 2D culture may require a significant investment in process development time as conditions must be validated for each vessel being considered for inclusion in the scale up strategy for manufacturing.
2) Timeline Considerations
Speed to market is an important factor to consider. In some cell therapy applications, where there is a race to be first to market, companies may opt to keep attachment dependent cell therapies in adherent manufacturing systems rather than spend time transitioning to a suspension-based system. In contrast, if
demand for the product is high, companies may choose to spend the time up front to transition to a suspension based system, if they determine that they can achieve higher and/or more cost- effective production by making the switch.
Another important consideration when deciding on a scalable manufacturing strategy is the product lifecycle. How long is the anticipated market relevance and demand for the product? What scale does production need to achieve to meet demand? The investment for a two- versus a ten-year product lifecycle will be very different and factors into capital investments for upgrading space and equipment or investing in a new technology platform. It is also prudent to ensure that the supply chain for critical components can support the product duration and demand to mitigate potential delays.
3) Facility Space and Labor Considerations
The available square footage for equipment can also impact scalability options. Real estate availability in many biotech hubs in North America can be scarce and expensive, making facility space a limiting resource for many cell therapy manufacturers. This can factor heavily into the adoption of certain cell culture platforms. Some culture systems are more compact than others, while others require additional equipment and/or skilled personnel to operate. In some cases, additional staff training will be necessary to implement new production technologies.
4) Budgetary Considerations
Many of the factors in determining the approach to scaling production of a cell therapy are impacted by budgetary constraints. How much capital investment can be put towards scaling processes – space, equipment, personnel, product demand and time available for process development and validation. Their associated cost must be weighed against the potential return before a decision can be made.

Navigating a Successful Tech Transfer
As Corning has worked with many cell therapy companies, we are able to provide an overview of the process of navigating a successful technology transfer. After thoughtful consideration of their current and likely future in-house manufacturing capabilities, many cell therapy developers opt to enlist the help of a contract manufacturing organization (CMO) or contract development and manufacturing organization (CDMO) to meet their production goals and timelines. The need for a GMP-manufactured product to meet regulatory requirements is also a driver to move production to a CMO/CDMO already equipped to operate in this space. This requires a transfer of knowledge from the sponsor company to the contract organization that often includes validation of the GMP process development and manufacturing methods to be used, implementation of a quality control strategy, and collaborating to secure supplier agreement plans and scheduling.
When choosing a CMO/CDMO, the careful selection of a service provider with extensive experience in large-scale manufacturing, knowledge of regulatory requirements and a commitment to collaboration is critical and can ease the technology transfer process. At Corning, our application scientists frequently facilitate the successful technology transfer from customers to their chosen CMO/CDMO. From their experience, they have identified the following key considerations when planning the transition.
1) Know your process and product
It is important to understand the critical quality attributes at small- scale that make your product work. Having analytical assays to characterize attributes like product identity, potency, purity and functionality is critical to the success of the technology transfer
process. These parameters help establish the quality control metrics against which the impacts of changes in the production process can be measured during scaling operations.
Also, early in the process it is important to communicate the projected demand for your product to determine whether the CMO/CDMO has the capacity to meet production requirements in the allotted timeframe. Ultimately, understanding key product quality attributes and timeline to market will facilitate a more informed decision when choosing a CMO/CDMO.
2) Find commonality with your CMO/CDMO
One of the key challenges facing tech transfer is the difference in equipment and operating procedures between the sponsor company and the CMO/CDMO. Problems can arise if the CMO/ CDMO selected is unfamiliar with the technology platform used by the customer, which would necessitate an adoption of processes that could cost valuable time and resources. Having some commonality with the CMO/CDMO, whether it is using a consistent cell culture surface (i.e., tissue-culture treated or Corning CellBIND® treated) and/or a consistent cell culture platform can mitigate risk in tech transfer. Having familiar touchpoints between the two sites can also be useful when problems arise and troubleshooting is required. For example, if the seed train from a T-flask to a Corning CellSTACK® worked for your process during development, keep it the same in tech transfer to the CMO/CDMO. Certain platforms, like the CellSTACK provide the opportunity to operate at varying scales depending on the vessel chosen, which can be advantageous.
After all, tech transfer is not a one-way street. It requires a dedicated team, continuous communication and collaboration for success. Enlisting the help of technology providers, like Corning, can provide significant value-add to the relationship between a customer and CMO/CDMO. The Corning team strives to unify and connect the two parties to ensure tech transfer success, such as facilitating training of technicians at both sites to ensure operational and performance consistency. Corning can also help CMOs/CDMOs identify economies between their different culture platforms to enable flexibility, decrease cost and streamline supply chain. As an example, the tubing manifold used in closed system operations with the Corning CELLStack 10-layer vessel are compatible with the Corning HYPERStack® 36-layer vessels, simplifying the number of parts that a manufacturer would need to stock, as well as negating additional staff training to operate the manifold between the two systems.
Ultimately, having a thorough understanding of the cell therapy product and leveraging the expertise of technology providers and contract organizations can streamline the scaling strategy to facilitate the transition from lab- to large-scale manufacture. Cell therapy developers looking to enter manufacturing scale with cell culture product specifications that are difficult to achieve at large scale will face costly delays. Therefore, it is prudent to keep scalability in mind as early as possible in the development pipeline to avoid this risk. The key to increasing patient accessibility to meet market demand for cell therapies will be better, scalable manufacturing processes in conjunction with automation tools that drive down cost and time-to-treatment.
Footnotes
-
1. Nianias A, Themeli M. Induced Pluripotent Stem Cell (iPSC)-Derived Lymphocytes for Adoptive Cell Immunotherapy: Recent Advances and Challenges. Curr Hematol Malig Rep. 2019;14(4):261-268. doi:10.1007/s11899-019-00528-6