
Semi-automated Closed CAR-T Manufacturing Process
CAR-T cell-based gene therapy technology is one of the most exciting new medical developments, with two of the most popular examples being the cancer immunotherapies Kymriah® and Yescarta®. These cell-based gene therapies have been able to provide unprecedented remission rates and have demonstrated success where other therapies have failed.
Even with impressive clinical success, there are challenges with the costly and complex manufacturing of these patient specific therapies. While much of the biotherapeutic industry is moving toward strategies to reduced cost such as single-use equipment, increased automation, and closed processes, most cell therapy manufacturing still contains open workflows and manual steps.
Automating and closing the manufacturing process can improve efficiency and product consistency. A closed process is a process where the biological material is never exposed to the open environment. By removing the exposure to the open environment, you can reduce the risk of contamination. Closed systems also enable increased automation, which can reduce timelines and cost. It can also permit better facility use in that you can have multiple patient samples in the same room and lower clean room requirements.
Achieving a completely closed system and automating it can be challenging as there is less fit for purpose equipment or kits designed for cell therapy use. In addition, fitting newly designed equipment into a fully integrated and closed system takes quite a bit of trial and error. Lastly, living cells are more complex and therefore more challenging to manufacture than other protein based biologics. These issues have made closing and automating a cell therapy manufacturing system more difficult.
A recent poster, “Development of a Semi-automated Closed CAR-T Manufacturing Process”, looks at designing a semi-automated closed system for manufacturing CAR-T cells. Authors from the Centre for Commercialization of Regenerative Medicine and GE Healthcare investigate individual CAR-T unit operations to identify commercially available reagents and modular equipment to drive process closure and automation that will improve workflow efficiency and product consistency.
Highlights of the poster include:
Authors set out to systematically build a semi-automated manufacturing process for CAR-T cell therapies that could be widely adopted by the T-cell immunotherapy industry. They wished to identify and utilize commercially available reagents that could enhance process efficiency. They also integrated automation strategies to reduce manual and open operations while at the same time maintaining the flexibility to concurrently manufacture CAR-T products. In Figure 1, authors demonstrate the method that they devised for manufacturing.
Method
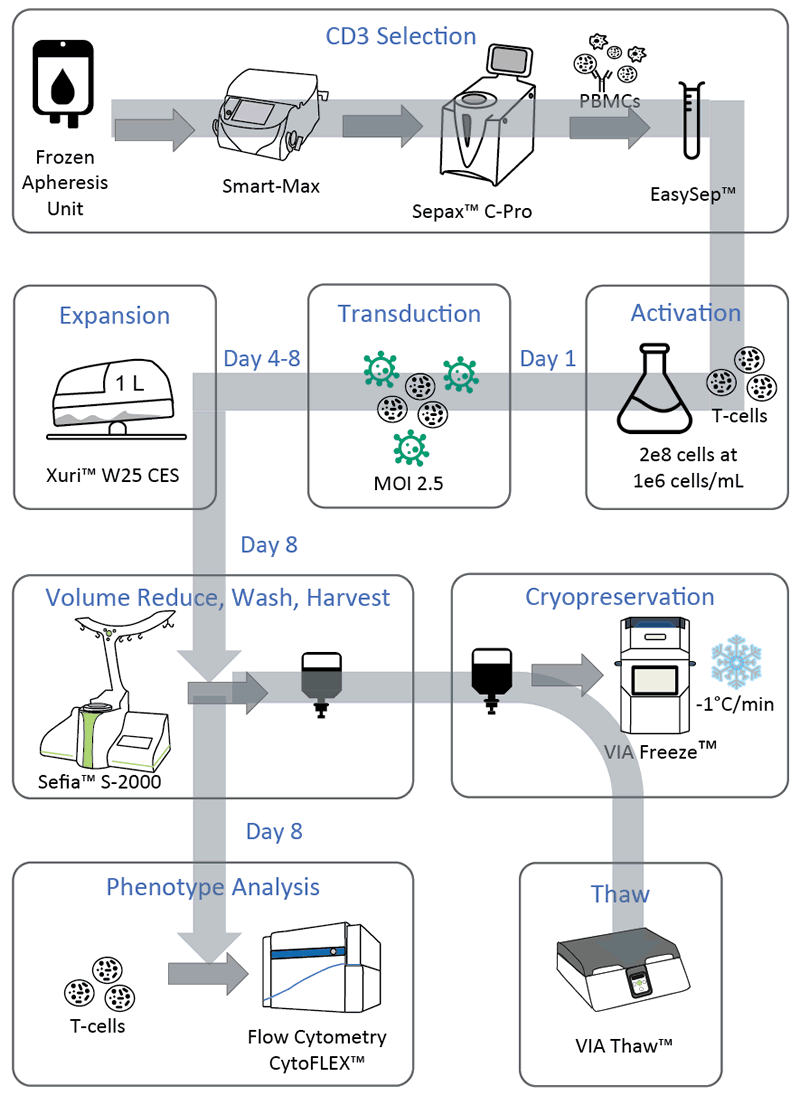
Results
Authors were able to develop a semi-automated closed CAR-T manufacturing. The system consists of 1E10 GFP transduced T-cells in an 8 day process using 2 donors. EasySepTM was identified as a commercially available reagent to enhance process efficiency the Smart-Max, SepaxTM C-Pro, XuriTM W25 bioreactor, SefiaTM and VIA FreezeTM were integrated to create a semi-automated process and reduce the number of manual operations. Simulated CAR-T manufacturing runs demonstrated the success of the identified method (Figure 1).
Simulated CAR-T Manufacturing Runs
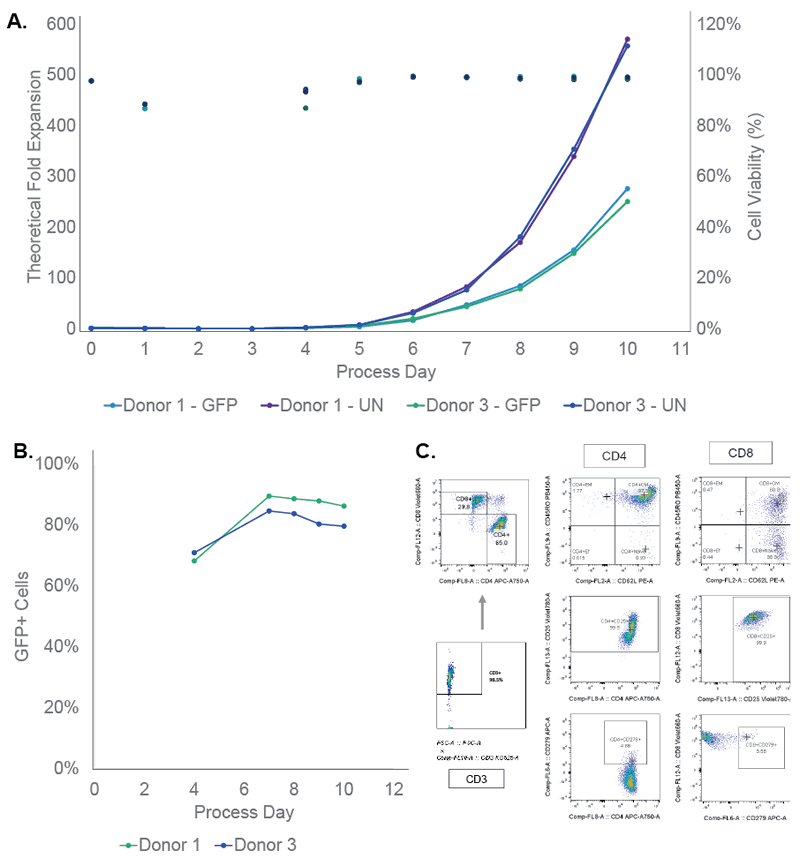
Summary
The resulting manufacturing process met the goals of being semi-automated and closed. The way that the process has been developed also makes it possible to be widely adopted by the T-cell immunotherapy industry. Authors stated that future work will include development of an isolation process with a closed EasySep™ platform, CAR-T manufacturing runs with transduction, expansion and harvest using patient material, further optimization of T-cell bioreactor expansion and integration of GMP grade reagents that are currently in development.
For more information and to view the data in full, please see the poster above.
Related Reading
CAR T Application Note – a further exploration of the poster data