
Solving the Solubility Challenge of Wnt Proteins with Gibco’s Wnt Surrogate-Fc Fusion Recombinant Protein
Wnt signaling is one of the key cascades regulating development and stemness during embryogenesis and is known to play a role in oncogenesis. Since their discovery, three Wnt signaling pathways have been characterized and all are activated by the binding of a Wnt-protein ligand to receptors belonging to the Frizzled (FZD) family and co-receptors such as lipoprotein receptor-related protein (LRP)-5/6. Wnt proteins are critical for numerous in vitro research applications ranging from stem cell differentiation strategies to the maintenance of epithelial stem cell–derived organoid models.
Great progress has been made to further our understanding Wnt signaling, but certain types of experiments have been hindered because Wnt proteins are lipodated, highly hydrophobic and insoluble. It is notoriously difficult to isolate pure Wnt proteins due to these physicochemical characteristics and purification typically involves the use of detergents to increase their solubility and minimize aggregation, but this can result in poor bioactivity and limit their use in certain in vitro and in vivo assays. Serum-stabilized Wnt3a conditioned media is a common substitute; however, the components of Wnt3a CM are not fully defined, introducing uncontrollable experimental variables.
The Gibco™ Wnt Surrogate-Fc fusion protein (Wnt surrogate) offers a novel solution to overcome the solubility challenge, which a major obstacle of working with Wnts. The Wnt surrogate is an engineered Fc fusion protein, which bridges the FZD and LRP5/6 receptors to activate Wnt signaling pathways with improved solubility and at much lower concentrations required compared to commercially available recombinant Wnt3a proteins or Wnt-conditioned media. The Wnt surrogate is composed of the LRP-binding domain of the extracellular Wnt modulator Dickkopf (DKK), linked to a designed ankyrin repeat protein (DARPin)—a selective, high affinity binder of Frizzled receptors (Figure 1). More specifically, this engineered Wnt mimetic interacts with FZD1, FZD2, FZD5, FZD7, and FZD8.
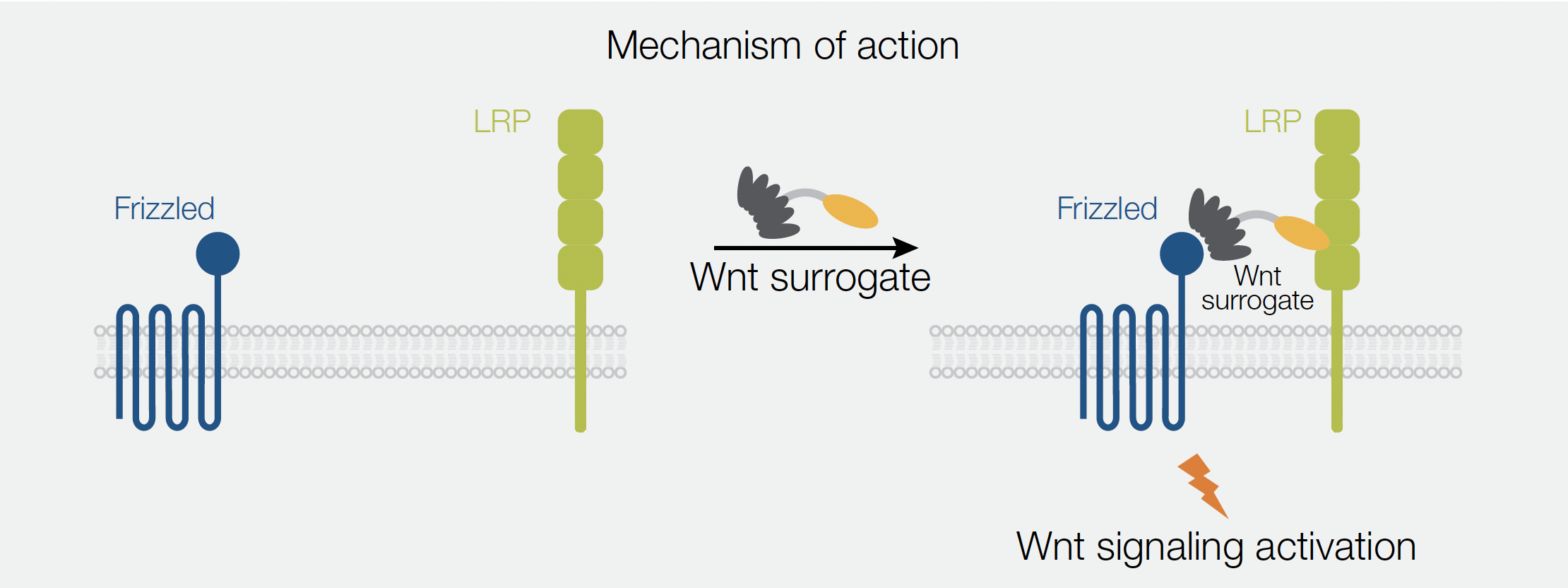
An application note released by ThermoFisher highlights the capabilities of the Wnt surrogate in Wnt reporter assays and demonstrate its ability to induce the expression of direct Wnt target genes in human induced pluripotent stem cells (iPSCs) in comparison to commonly used Wnt agonists.
Wnt Reporter Assays
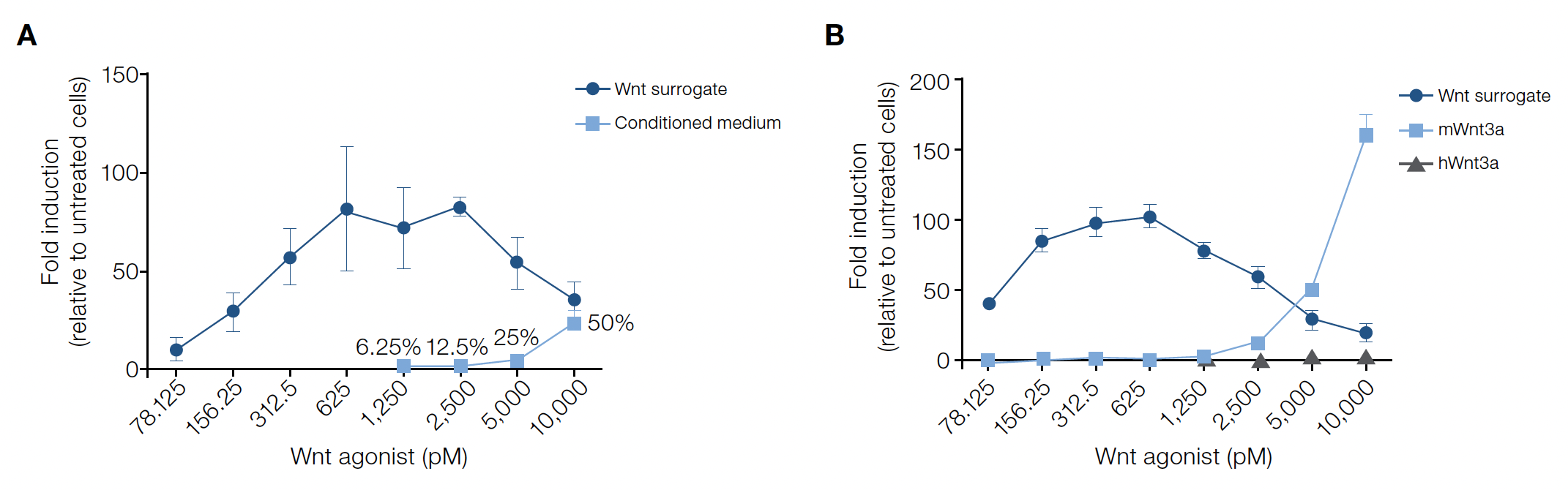
The Topflash assay is widely used to measure Wnt signaling and consists of Wnt-responsive DNA elements that control the expression of a reporter (i.e., luciferase). To characterize the activity of the Wnt surrogate, adherent HEK293T reporter cells were treated with varying concentrations (0–0.625 nM) of the fusion protein for 18–20hrs prior to quantification using luciferase assays.
The Wnt surrogate exerts a dose-dependent activation across the picomolar (pM) to nanomolar (nM) range and at concentrations of ~0.156–0.625 nM, the fusion protein was a strong agonist. The activity profile was found to be consistent across multiple manufactured lots of the Wnt surrogate with the half-maximal effective concentration (EC₅0) range determined to be 0.068–0.106 nM in the Topflash assay.
In experiments directly comparing the Wnt surrogate to commonly used Wnt agonists, signaling activation can occur at much lower concentrations. At ~100–5,000 pM, the Wnt surrogate activated the reporter more strongly than 6.25–50% v/v Wnt3a conditioned medium (Figure 2A) and nano- to micromolar concentrations recombinant mouse or human Wnt3a (Figure 2B). The Wnt surrogate can achieve potent activation at reduced concentrations, offering researchers an alternative cost-effective Wnt pathway agonist for use in a number of model systems.
Wnt Surrogate for Pluripotent Stem Cell Differentiation
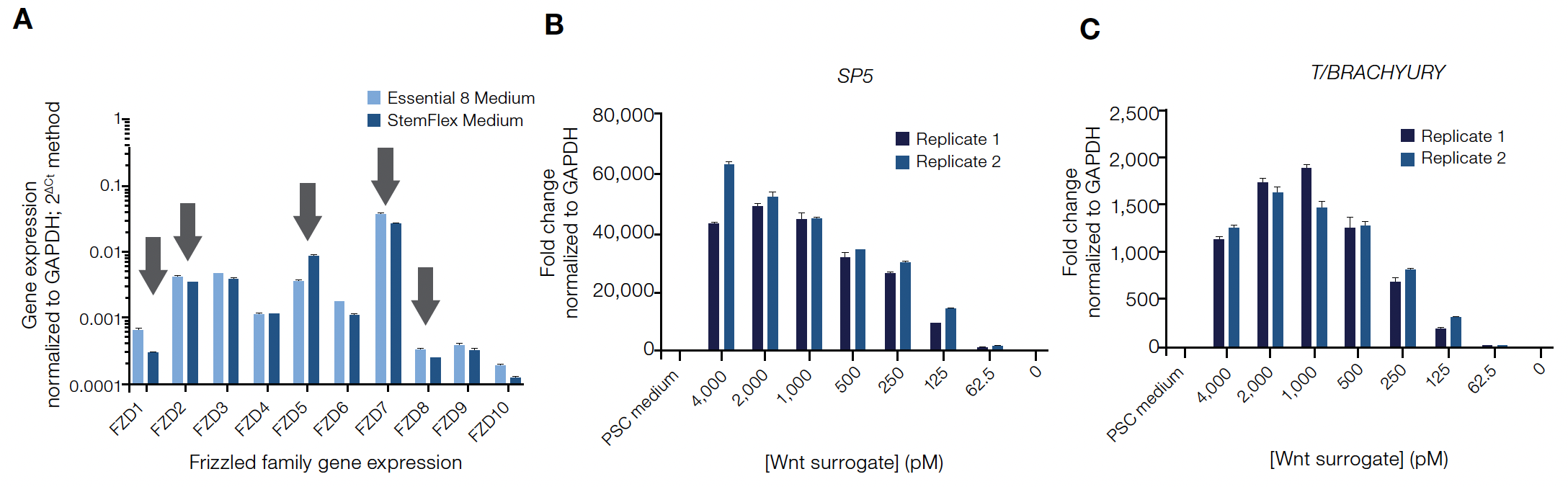
Wnt agonists are commonly used to direct the differentiation of human induced pluripotent stem cells (hiPSCs) into mesoderm and other cell lineages. Because the Wnt surrogate functions through a subset of Frizzled receptors, gene expression patterns of the Frizzled (FZD) family were first confirmed in undifferentiated hiPSCs before experiments to evaluate its ability to direct differentiation. In two different PSC culture systems, similar FZD family expression profiles were measured including robust expression of Wnt surrogate-specific FZD receptors indicating these cells should be responsive to the Wnt surrogate (Figure 3A).
To analyze the Wnt surrogate’s activation potential for Wnt target genes, undifferentiated Gibco™ hiPSCs were treated across a range of concentrations before analyzing the cells for expression of key Wnt target genes using qPCR (Applied Biosystems™ TaqMan® Assays). The Wnt surrogate was able to activate endogenous Wnt target genes SP5 and T/BRACHYURY in a dose-dependent manner—genes known to be associated with early germ layer differentiation (Figure 3B and C).
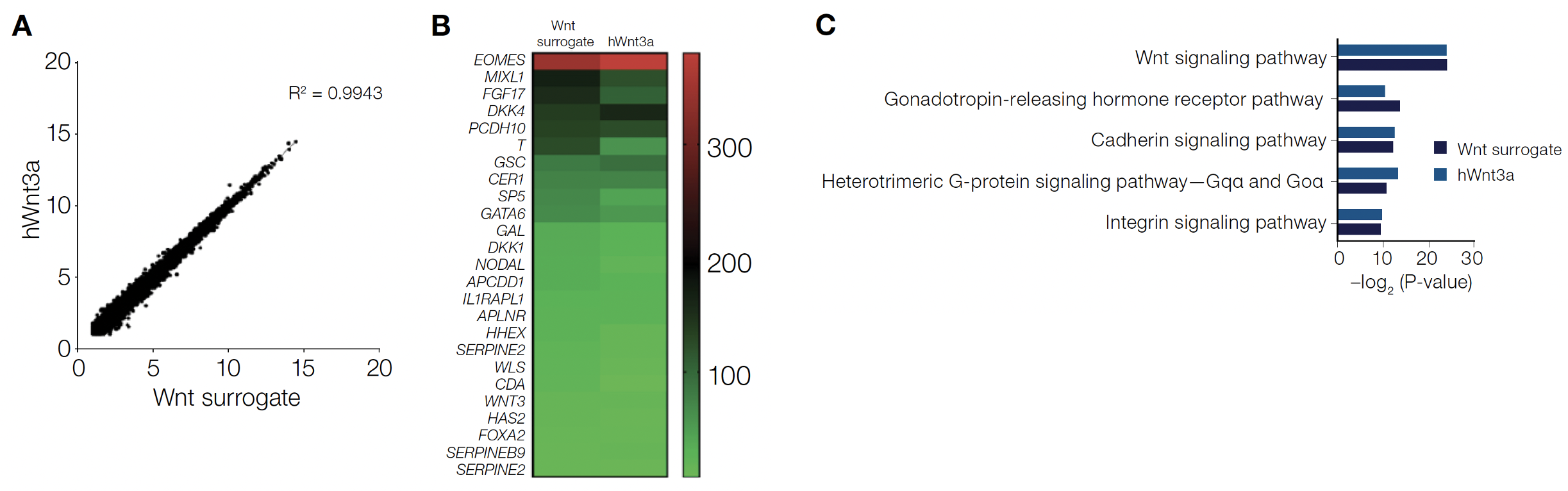
Additional characterization and evaluation of the Wnt surrogate compared to human and mouse recombinant Wnt3a ligands and the small-molecule GSK3β inhibitor CHIR99021 are described in detail in the application note. Next-generation sequencing shows the Wnt surrogate can recapitulate the Wnt3a global transcriptional response in hiPSCs. Further gene ontology analysis shows the Wnt surrogate transcriptome is strongly correlated with profiles generated using other Wnt3a agonists and most closely resembles that of wild type–based recombinant hWnt3a (Figure 4). The Wnt surrogate and hWnt3a modulate were shown to modulate the same gene pathways in human iPSCs with a high degree of similarity. The activity of the Wnt surrogate at 100–500 pM was equivalent to 2.4–2.5 nM (i.e., ~100 ng/mL) of the hWnt3a. All the data suggests the Wnt surrogate is a true Wnt mimetic and can effectively replace hWnt3a in existing protocols but at much lower concentrations.
Together, the results from the comprehensive testing of the Wnt surrogate is a mimetic Wnt agonist with potent activity and comparable performance to widely used Wnt agonists such as human or mouse recombinant Wnt3a, Wnt-conditioned medium, and the GSK3β inhibitor CHIR99021, at significantly lower concentrations. The Gibco™ Wnt surrogate-Fc fusion protein is a highly potent and soluble Wnt pathway activator that overcomes known limitations of commercially available recombinant Wnt ligands and may be a useful addition to the laboratory “tool-kit” to advance the study of Wnt pathway signaling in development and disease.