
Spheroid Models: Getting Started and Culture Tips
“3D Model Systems: Spheroids, Organoids and Tissue Models”.
You can download all the articles in the series, by downloading the eBook.
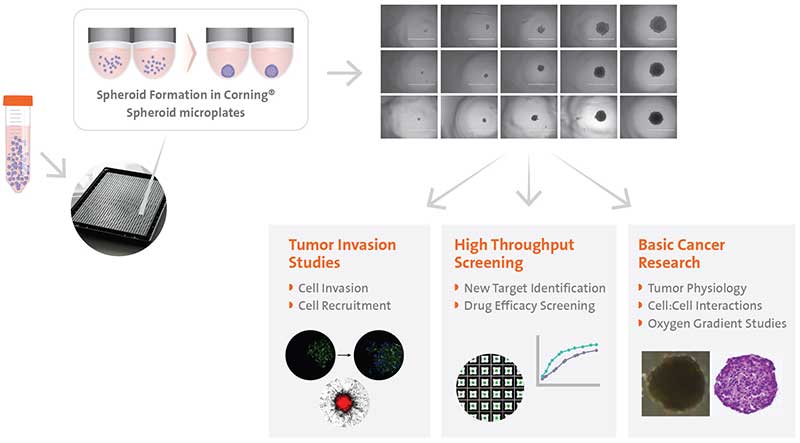
It has been well established that three-dimensional (3D) cell culture is better at replicating the physiological interactions between cells and their environment than traditional two-dimensional (2D) cell culture. In fact, many types of mammalian cells have the ability to self-aggregate into 3D aggregates called cellular spheroids when cultured in suspension or in a nonadherent environment. Common examples of spheroids include embryoid bodies, mammospheres, hepatospheres, and neurospheres. Perhaps the most widely utilized spheroid model is the multicellular spheroid model (MCTS) formed from cancer cells, which is commonly used as avascular tumor models to gain mechanistic insight into cancer invasion and metastasis and as an anti-cancer therapeutic screening tool. The heterogeneous population of proliferating and non-proliferating cells is exposed to a gradient of oxygen emulating the physio-chemical gradients found in solid tumors, therefore, making them more predictive in vitro models than 2D cultures. Additionally, they are also relatively easy, fast, versatile, and are inexpensive enough to enable high-throughput screening, which makes them attractive models for many applications (Figure1).
Spheroid Generation
SCAFFOLD-FREE METHODOLOGIES
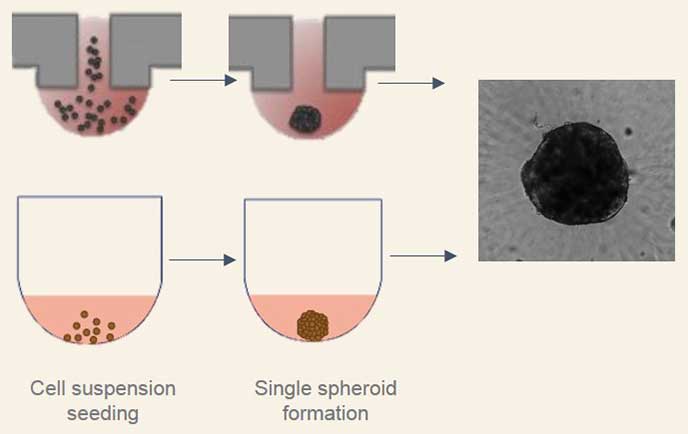
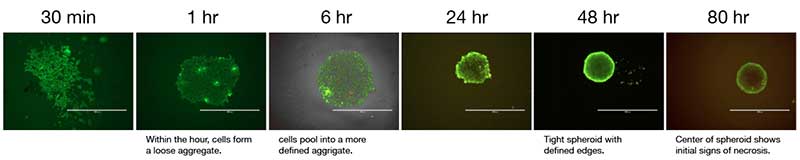
Scaffold-free methods are commonly used to generate spheroids where cells are cultured to prevent adhesion and promote aggregation such as hanging drop technology, rotary cultures, and bioreactors or the use of specialized non-adherent cell cultureware like the Corning® Ultra-Low Attachment microplates. Traditional methods such as the hanging drop for spheroid formation take advantage of cell sedimentation in droplets of cell culture medium to promote cell aggregation. While this method is effective, it can be time-consuming and difficult to execute reproducibly. Corning® spheroid microplates have opaque side walls, a unique clear round well-bottom design, and an Ultra-Low Attachment (ULA) surface coating that is hydrophilic, biologically inert, and non-degradable, which enables the rapid and highly reproducible formation and growth of a single spheroid per well. When these two methods were used to form human liver microtissue spheroids, the spheroids produced were comparable in size, longevity, and functional tissue characteristics (Figure 2).
Currently, Corning spheroid microplates are available in 96-well (Corning Cat. Nos. 4520 and 4515), 384-well (Corning Cat. Nos. 3830, 3830BC, and 4516), and 1536-well (Corning Cat. No. 4527) formats.
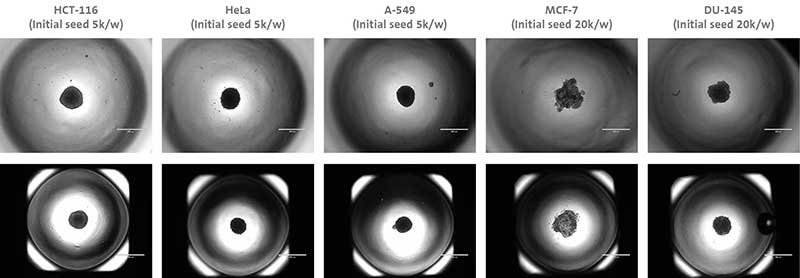
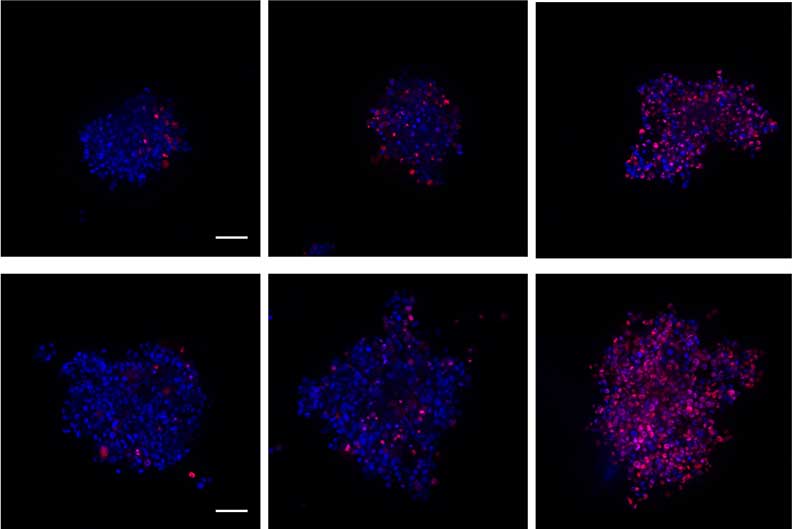
Below (Figure 3) are some examples of different cell types forming spheroids using different Corning® microplate formats.
HIGH THROUGHPUT SCREENING
In order to meet the demands of most drug discovery operations, a high throughput system is essential to success. The Corning® 1536-well spheroid microplate allows the formation of 1536 uniform, single spheroids that can be assayed via imaging, fluorescence, or luminescence directly in the microplate. With a maximum volume of 14 μL per well, accurate liquid handling is essential to the generation of quality data. Corning® has established an automated method for seeding, dosing, and assaying spheroids in a 1536-well microplate format using INTEGRA Biosciences liquid handling instruments.
The VIAFILL is designed for rapid bulk liquid dispensing at volumes as low as 0.5 μL and can be fully automated with a plate stacker accessory
The VIAFLO384 is a handheld electronic pipettor which enables transfer into 24-, 96-, 384-, and 1536-well microplates in a fast, compact, and easy-to-use manner
Helpful Tips
When using the Corning® spheroid microplates, the size of the spheroids is dependent on factors such as initial plating densities, cell-type, duration of growth phase in a spheroidal format and the desired size of spheroid at the time of assessment. A single cell suspension is required to seed into the microplates. If the cells used are particularly sticky, a 40 μm cell strainer may be required to achieve a single cell suspension. If cells are seeded manually, care should be taken to avoid touching the bottom or sides of the microwells with pipet tips to avoid damaging the ULA surface coating. The maximum working volumes for each microplate format are as follows:

Many cell lines will form spheroids within a 24-hour period. However, some may require a 15-minute incubation post-seed or centrifugation at low speed (i.e. 150 x g for 5 mins) to promote cellular aggregation. The addition of media supplements such as methylcellulose or other cell types such as fibroblasts can help with cells that tend to form looser aggregates as opposed to spheroids. Optimization of the protocol is recommended for your cell type of choice.
Depending on the cell type and the duration of the spheroid culture, a media exchange step may be required. In order to leave spheroids undisturbed during media changes, sides of wells should be used to remove and add media. It is recommended that 10 to 20 μL volume be left in the well for the 96-well and 384-well formats and 5 μL for the 1536-well format so the spheroids are not left dry during the media change. Automation will be required for liquid handling of the 1536-well format.
Imaging and Staining Spheroids
Another advantage to using the ULA microplates is that spheroids may be generated, cultured, and assayed for fluorescent or luminescent signals in the same plate without the need to transfer the spheroids. The unique well geometry and opaque side walls of Corning® spheroid microplates reduces background fluorescence/luminescence, making them suitable for automated imaging of spheroids for high content screening approaches. The plate design also includes a unique well shield to minimize well-to-well cross-talk.
Helpful Tips
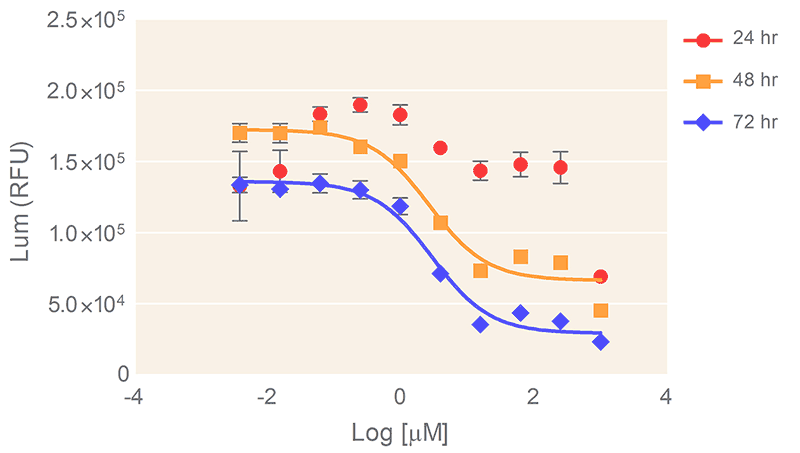
The Promega CellTiter-Glo® 3D Cell Viability Assay (Promega Cat. No. G9683) for cell proliferation and cytotoxicity assay screening is recommended. Figure 6 shows results from cell proliferation and cytotoxicity assay of the human prostate cell line DU145 in response to Doxorubicin treatment.
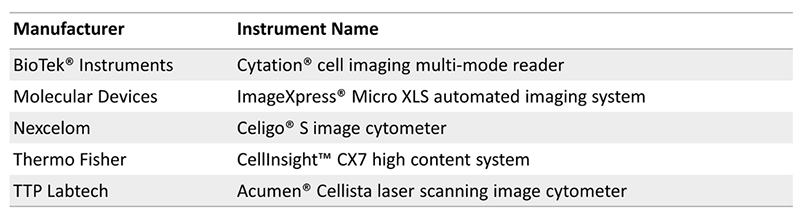
There are a number of commercial instrumentation compatible with spheroid microplates:
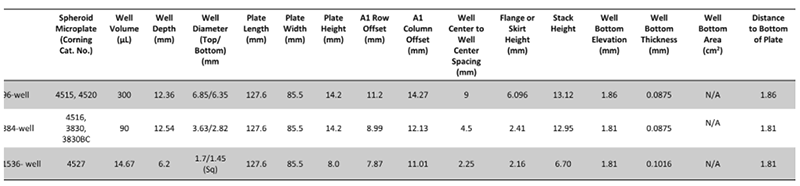
The dimensions of the Corning® microplate formats are listed below for easy instrumentation setup:
Oncology Models
The Corning® HTS-Transwell permeable supports (Corning Cat. No. 3387) are synthetic scaffold inserts with microporous membrane bottoms that can be fitted on the spheroid microplates. The membranes of these supports are available with small pore sizes (i.e. 0.4-8.0µm micron) that permits movement of molecules and/or the cells themselves across the membrane, which are located on opposite sides of the membrane. This format allows researchers to investigate cellular interactions and is amenable to tumor invasion studies.
CO-CULTURE MODELS
The interaction of tumor cells with other cell types in the tumor microenvironment can be one predictor of a possible therapeutic outcome. For example, evidence shows stromal cells induce chemo-resistance, protecting tumor cells from the toxic effects of anti-cancer drugs. Endothelial cells and the associated vasculature provide blood supply to the tumor allowing for its proliferation but are also responsible for carrying therapeutic compounds to the cancer cells. Therefore, the ability to model these cellular interactions in
vitro using 3D co-culture models is very useful to study complex interactions between cancer cells and ancillary cell types to determine their impact on tumor growth, vascularization, metastasis and response to chemotherapeutic agents.
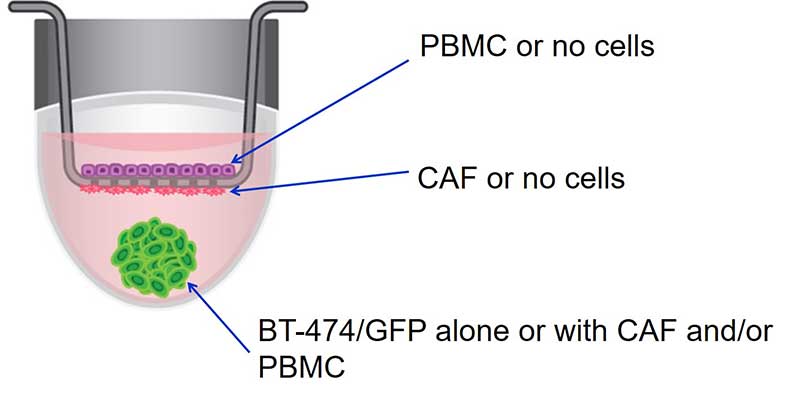
In one such study, breast cancer cells co-cultured with stromal cells with and without direct contact was studied to determine if these cellular interactions affected the response of the cancer cells to therapeutic compounds. When looking at direct cell interactions, breast cancer-associated fibroblasts (CAF), peripheral blood mononuclear cells (PBMCs) and cancer cells from the GFP-expressing breast cancer cell line BT-474 were added directly to spheroid microplates and cultured. To look at indirect interactions, the stromal cells were seeded onto the Corning® HTS Transwell-96 inserts which was seated on top of a spheroid microplate. The BT-474 cells were seeded into the spheroid microplate, separated from the stromal cells by the Transwell inserts with a 1μm pore size. The viability of the BT-474 cells was assessed before and after exposure to a chemotherapeutic agent in the control BT-474 monoculture, the direct and indirect co-cultures (Figure 7).
TUMOR INVASION STUDIES
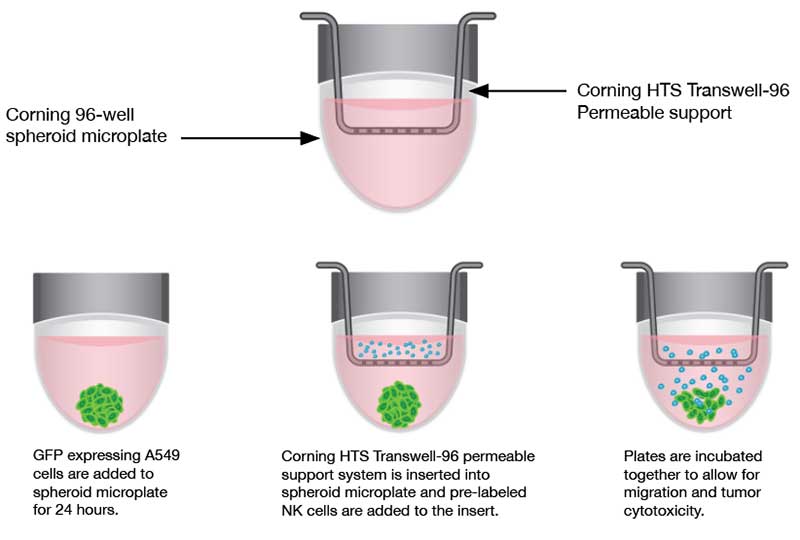
Traditionally, tumoricidal activity and immune evasion have been studied by utilizing 2D systems, which may not accurately reflect the complexity of a tumor in a 3D environment. The Corning® spheroid microplates to generate uniform 3D spheroids combined with Corning® HTS-Transwell inserts enable researchers to investigate immune cell homing, tumor cytotoxicity, and tumor immune evasion in an easy-to-use 3D high throughput assay (CLS-AN-425). In one study, the ability of natural killer (NK) cells to infiltrate tumor spheroids generated from A549 cells (adenocarcinoma human alveolar basal epithelial cells) was examined. Figure 8 shows the workflow for the migration and tumor cytotoxicity assays.
Undoubtedly, 3D spheroid cultures are an important addition to the research toolbox with huge translational potential, particularly for cancer and stem cell research. Their physiological relevance and the predictive nature of spheroids is helping to bridge the gap between in vitro and in vivo models, helping to drive novel discoveries and innovation.