Enhance Your Cell Culture Performance with Chemically Defined Peptides
Animal cell culture is a central process for successful bioproduction of therapeutic proteins, viral vectors (for vaccines and gene therapies) and therapeutic cells (for cell-based therapies), which help prevent, treat and cure human disease. Optimized cellular nutrition is the key to unlocking cost-effective, highly efficient bioprocesses. Amino acids are essential nutrients and critical ingredients in cell culture media required for in vitro cell survival and proliferation because of their role as building blocks for proteins. Standard practice in biopharma is to add free amino acids to media formulations based on the nutritional requirements of the cell type of interest. However, the stability and solubility of certain free amino acids have quickly become limiting factors to higher productivity requirements as part of industrialized biomanufacturing (i.e., fed-batch or perfusion culture). Optimal media composition with the best ingredients at the right concentration helps drive productivity gains, streamline workflows, and reduce operating costs.
Current Challenges
Some of the key amino acids required for biomanufacturing have properties which pose barriers to intensification strategies for enhanced productivity. For example, L-glutamine is unstable at physiological pH in liquid media, breaking down to ammonium and pyroglutamate that can be problematic for many biomanufacturing applications. Other amino acids like L-tyrosine and L-cystine, which exhibit low solubility at neutral pH, are at risk of precipitation at higher concentrations. With a solubility of less than 0.5 g / liter in water at neutral pH, L-tyrosine is particularly difficult in concentrated feed media stocks, which are often used to replenish nutrients throughout the culture period in both fed-batch and perfusion culture systems. Stability and solubility challenges limit the amino acid bioavailability and their suboptimal concentrations in bioreactor feed or perfusion media can directly impact cellular productivity resulting in low-performing bioprocesses.
Current Solutions Miss the Mark
Some mitigation strategies exist to overcome current challenges with free amino acids, but they can increase costs, add extra processing steps, and prolong the culture period. For instance, to deal with the instability of L-glutamine, it is common to add L-glutamine fresh to culture and feed media to avoid degradation. While this can prevent degradation to a certain extent, it complicates overall processing and may still result in an undesirable accumulation of by-products and potential toxicity.
In fed-batch, the pH of the concentrated feed media is often altered (alkaline conditions) to increase the solubility of amino acids like L-tyrosine and L-cysteine, but this creates the need for complex control strategies to minimize pH spikes or gradients during feed additions and mitigate the risk of precipitation. This approach also increases osmolality through multiple pH shifts and cannot be used to increase solubility in perfusion media or inside the bioreactor.
The industry needs better solutions to overcome the core challenges associated with free amino acids.
cQrex® Peptides Offer a Solution
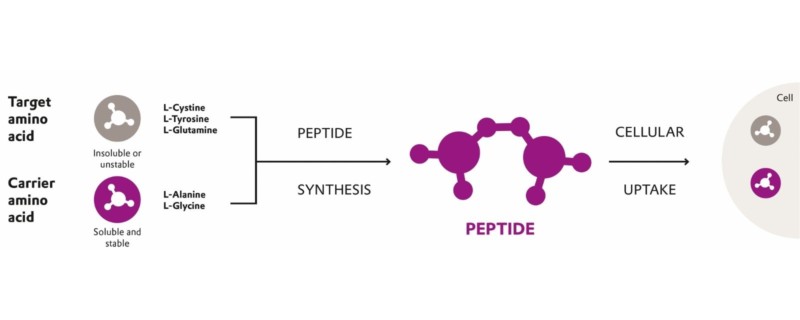
The Evonik cQrex® portfolio of chemically defined peptides is an innovative solution to replace free amino acids in biopharma cell culture media. Peptides are formed through chemical coupling of a target amino acid (i.e., L-glutamine) to a carrier amino acid resulting in stable and soluble peptide. Upon cellular uptake, the peptide is metabolized by the cell, making the free amino acids available. Based on glutamine, tyrosine and cystine, the cQrex® ingredients are non-animal derived, highly pure additives, manufactured at well-established ISO-certified, cGMP facilities in Europe and designed to enable high-performance commercial bioproduction. They provide the enhanced solubility and stability characteristics required for optimized nutrient supply and support intensified and simplified manufacturing of high-quality therapeutics with increased cell-specific productivity.
For more information on the Evonik cQrex® ingredients, please visit www.evonik.com/cQrex or contact us directly on cQrex@evonik.com
For webinars, product brochures and samples, please visit https://oncare.evonik.com/