Transfection Reagent Portfolio Enables Seamless Scalability from R&D to Commercial Therapeutic Viral Vector Manufacturing
Cell and gene therapies are achieving greater success in the clinic and as a result there is an increasing need to transition manufacturing from research scale to clinical and ultimately to commercialization. Critical to the manufacture of many gene-based therapies is the production of large quantities of recombinant viral vectors. Two commonly used recombinant viral vectors, Adeno-Associated Virus (AAV) and lentivirus, are mainly produced using PEI-mediated transient transfection in adherent and suspension HEK-293 or HEK-293T cells.
One common challenge in viral vector production is transitioning from research-based production to more rigorous quality and safety profiles for clinical and commercial manufacture. In fact, raw materials used in production at research scale are often not cGMP-compliant and therefore fit for clinical or commercial manufacturing. This means that critical raw materials frequently need to be replaced with cGMP grade material sourced from approved suppliers during process scale up. cGMP grade materials are manufactured under strict conditions and must pass rigorous testing. This higher manufacturing standard reduces the risk of introducing adventitious agents into clinical and commercial viral vector manufacturing.
To ease challenges with scale up, Polyplus-transfection® now offers their PEIpro®, PEI-based transfection reagent, in different quality grades, allowing a seamless transition from process development with PEIpro®-HQ to cGMP biomanufacturing with PEIpro®-GMP. A recent poster, “High Quality Transfection Reagents for Therapeutic Virus Production,” demonstrates the productivity and scalability of their product portfolio as well as the way that the different quality grades provide easy transition from research production to clinical and commercial requirements.
Highlights from the poster include:
Productivity
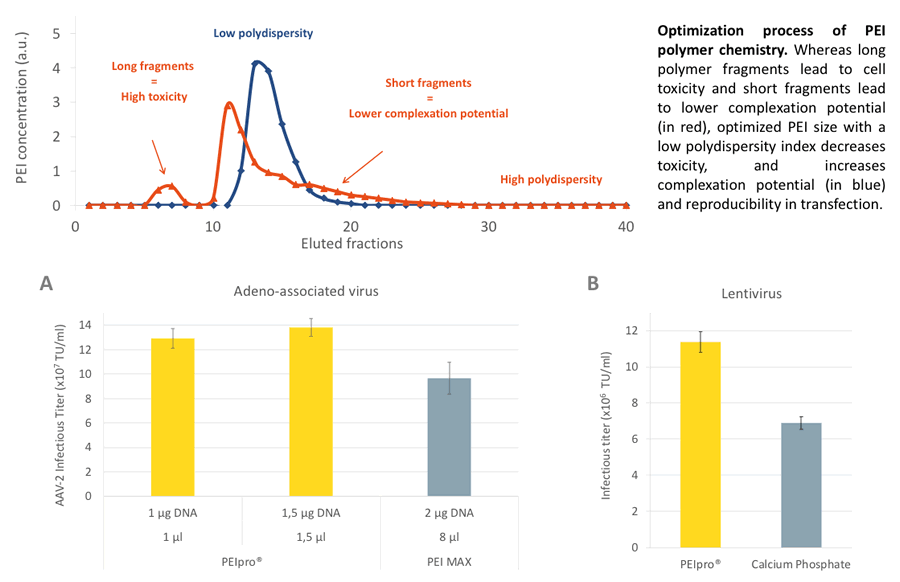
In the poster, Polyplus-transfection® describes the optimization process of their PEI polymer chemistry. They have created an optimized PEI size with a low polydispersity index that increases complexation potential and reproducibility in transfection with minimal toxicity (Figure 1).
PEIpro® was also shown to produce more AAV and lentivirus with less reagent and lower DNA amount when respectively compared to PEI MAX and Calcium Phosphate transfection (Figure 1).
Optimized transient transfection for virus production
Flexibility
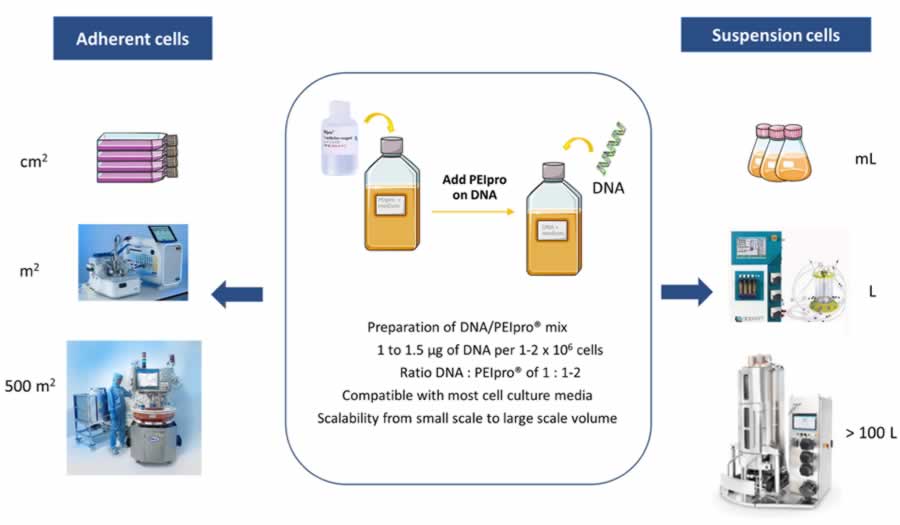
PEIpro® works well in both adherent and suspension cultures, in different culture vessels and at different scales (Figure 2). In the poster, authors share data provided by Généthon to demonstrate the use of PEIpro® in both AAV and lentivirus production in HEK-293T and HEK-293 cells grown in suspension bioreactors. They also share data provided by Pall, which highlights the use of PEIpro® for AAV-8 production in in the iCELLis fixed-bed Nano bioreactor (see poster for data).
Efficient virus production in any system at any scale
Scalability
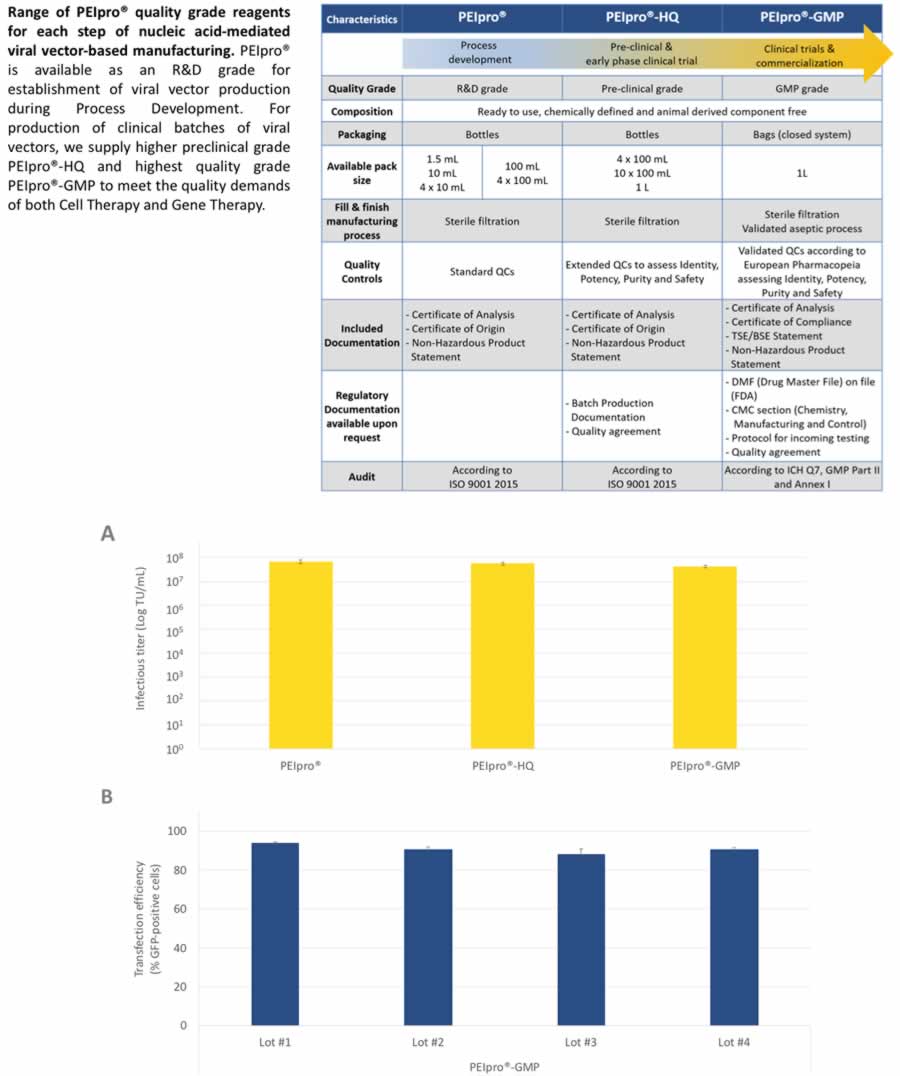
In the poster, the full portfolio of PEIpro® quality grade reagents is described. PEIpro® is available at R&D grade for establishment of viral vector production during Process Development. For production of clinical batches of viral vectors, there is a higher preclinical grade PEIpro®-HQ and the highest quality grade PEIpro®-GMP is available for therapeutic grade manufacturing (Figure 3).
The high reproducibility of PEIpro® irrespective of the quality grade and the production lot is presented through data in suspension HEK-293T culture using all grades and multiple lots of PEIpro®. The data presented shows similar productivity and efficiency across all grades and lots, thus making the scale up from R&D grade material to clinical and commercial grade material simple and straightforward (Figure 3).
Seamless transition from process development up to clinical trials and commercialization
To see the data in full, please click on the poster at the beginning to view in full size.
To learn more https://www.polyplus-transfection.com/products/peipro-product-range/