
Upstream Continuous Process – Perfusion Culture
Continuous processing has proved a very successful model in many other industries. As such, there has been a growing interest in utilizing continuous process concepts in the manufacture of biopharmaceuticals. Furthermore, certain continuous technologies have already been incorporated into existing biopharmaceutical manufacturing with many benefits. For example, in several applications, perfusion culture has provided benefits over traditional fed-batch processing and has been used successfully for many years to manufacture unstable proteins. These benefits can include higher yield, increased speed, cost savings, efficient facility utilization, better scalability and improved product quality and stability.
While implementing an end-to-end continuous process in biopharmaceutical manufacturing may still be many years away for most companies, there are some real advantages in utilizing portions of a continuous process in certain areas of manufacturing. Continuous processing technologies can provide solutions to specific challenges and can drive the implementation of a continuous or semi-continuous process.
At BPI West this year there were many talks on continuous processing technologies in both upstream and downstream. Over the next few weeks, we will have more articles on both continuous processing overall and specifically in downstream, but for this piece I will focus on some of the available upstream continuous process technologies.
Upstream Continuous Process – Perfusion Culture
At this time, perfusion culture is the only culture method that can support continuous processing in upstream. Perfusion bioreactors culture cells over much longer periods, even months, by continuously feeding the cells with fresh media and removing spent media while keeping cells in culture. In perfusion there are different ways to keep the cells in culture while removing spent media. One way is to keep the cells in the bioreactor by using capillary fibers or membranes, which the cells bind to. Another does not bind the cells, but rather relies on filtration systems that keep the cells in the bioreactor while allowing the media to be removed. Another method is the use of a centrifuge to separate cells and return them to the bioreactor.
Perfusion Culture Applications
As previously mentioned, a process can be fully continuous or semi-continuous and many groups are adding continuous process technologies to key areas of manufacturing in order to improve efficiency, solve issues with facility fit, create a multiple product facility, protect sensitive proteins, etc. Another strategy for using an upstream continuous process can be for developing fast, small, and scalable manufacturing for regulatory and toxicology studies. This provides a real advantage in terms of speed if a candidate were successful in initial studies.
Perfusion Culture Benefits
While the benefits of perfusion culture really depend on the specific application, there are some areas where perfusion can have an advantage over fed batch processes.
These areas include:
- Stability – Perfusion culture has a proven track record of success in manufacturing unstable proteins including coagulation factors and enzymes.
- Product Quality – A key advantage here is that you can maintain high cell density and optimal steady state conditions, thereby lowering levels of impurities and enabling high and consistent product quality. In addition product quality is improved because of decreased product hold time and there is less intermediate testing needed. Ideally perfusion systems are run as closed systems, which minimizes bioburden risk and these systems are usually compatible with QbD and PAT methodologies.
- Flexibility – Perfusion bioreactors are smaller in size and are typically compatible with disposable products. This enables rapid capacity increase or decrease with little impact to overall facility footprint. This size advantage is important because it means that facilities don’t need a significant increase in space to increase production.
- Less manual operations and a high level of automation – Automation reduces the amount of manual operations, thus freeing operator time for other tasks and reducing the risk of operator error.
Perfusion Culture Technologies
Hollow Fiber Bioreactors
One type of perfusion culture that makes a good fit with a fully continuous process was presented during the BPI West talk titled, “Hollow Fiber Bioreactors: Single Use. Perfusion. Scalable. Continuous Manufacturing.” The talk given by Scott Waniger, Vice President, BioServices, Cell Culture Company, described how hollow fiber bioreactors work and the manufacturing advantages they offer, which I have summarized below.
Hollow Fiber Technology
Hollow fiber perfusion bioreactors contain thousands of tube-shaped, semipermeable capillary membranes called hollow fibers. The hollow fibers are packed together in a cylindrical cartridge with an inlet at one end of the cartridge and an outlet at the other. The fibers are packed in a way that allows cells to be seeded and grown in the extracapillary space (ECS) outside the fibers. Medium continuously flows through the inside of the hollow fiber in the intracapillary space (ICS). By utilizing the hollow fiber membrane’s molecular weight cut off, typically between 10-100 kDa, you can control the flow of molecules. Thus, small molecule nutrients and oxygen are able to flow through the fibers and be delivered to the cells, while waste travels across the fibers to the intracapillary space to flow out. Importantly, the molecular weight cut off keeps the larger molecular weight products including monoclonal antibodies, recombinant proteins, viruses or whole cells to be held within the extracapillary space. Spent media can then be removed and replaced with fresh media or oxygenated and returned. The flow of nutrients and waste in and out of the cells paired with the 3D culture configuration creates a considerably more in vivo-like environment, allowing the growth of cells to very high densities, in the range of 109 cells/ml.
Key Cell Culture Advantages
There are several advantages of the hollow fiber bioreactor system that allow for enhanced cell growth. Perhaps most prominent is that cells are allowed to grow in 3D culture at very high densities, which provides more in vivo-like conditions and healthier cells. This type of culture allows cells to maintain high cell density and viability for extended periods, which leads to very consistent culture and product harvest.
Key Manufacturing Advantages:
- High Density Culture – at > than 109 cells/ml of space.
- Scalability – Another advantage is that hollow fiber technology is very scalable. Once the process requirements for one cartridge are determined during process development, the process scales linearly, quickly, and predictably based on higher numbers of like cartridges in use. While the size of the hollow fiber cartridges themselves don’t change, additional cartridges can be added in parallel to increase scale. If demand decreases, run times can be shortened and equipment can be taken off-line or moved to another project. (Figure 1)
- No Seed Train – once cells have been scaled up to 0.5-3 liters of cells they can be injected directly into the hollow fiber bioreactor EC space where they will continue to expand and grow to high densities.
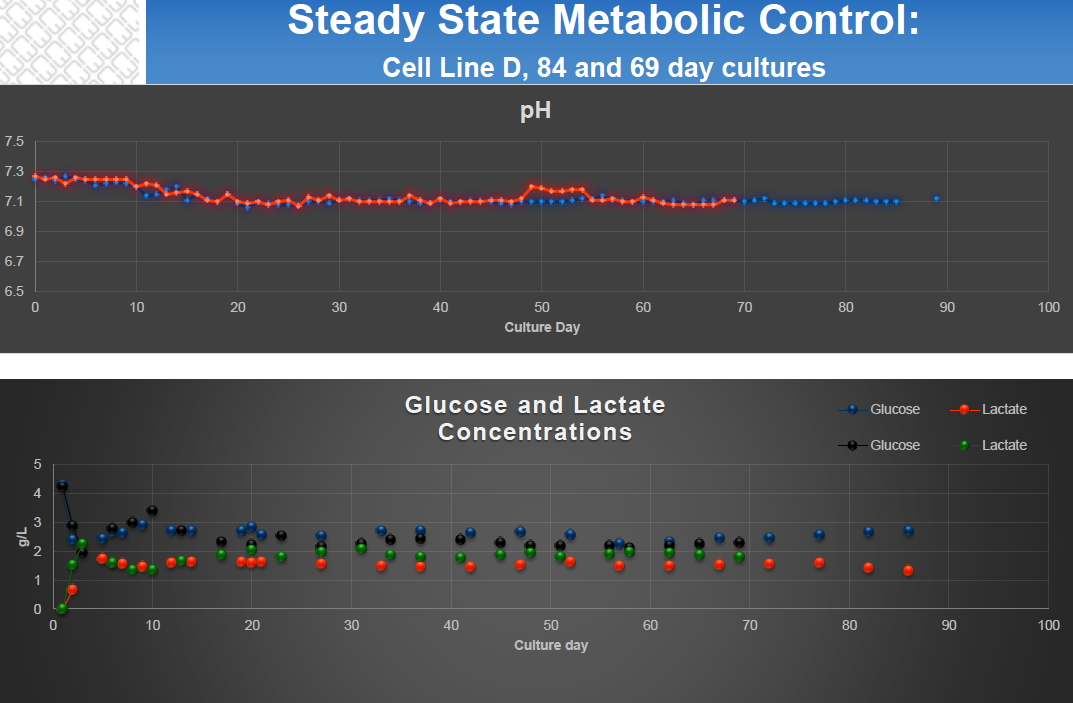
- Steady State Metabolic Activity (Figure 2)
- Long Lasting Protein Expression – Hollow fiber culture is a continuous perfusion process with typical runs being 60-180 days and product can be obtained through continuous or batch harvests. (Figure 3)
- Automation – most hollow fiber bioreactors support automation, which greatly reduces daily maintenance in comparison with other manufacturing systems. Most systems require only 20-30 minutes daily due to automated monitoring and control of pH, gassing, temperature, lactic acid levels and rate of nutrient delivery.
- Simplified Downstream – because of the confinement of cells to the extracellular space, product concentration of 100x or higher can be achieved thus eliminating the need for centrifugation and clarification steps downstream.
Figure 1:
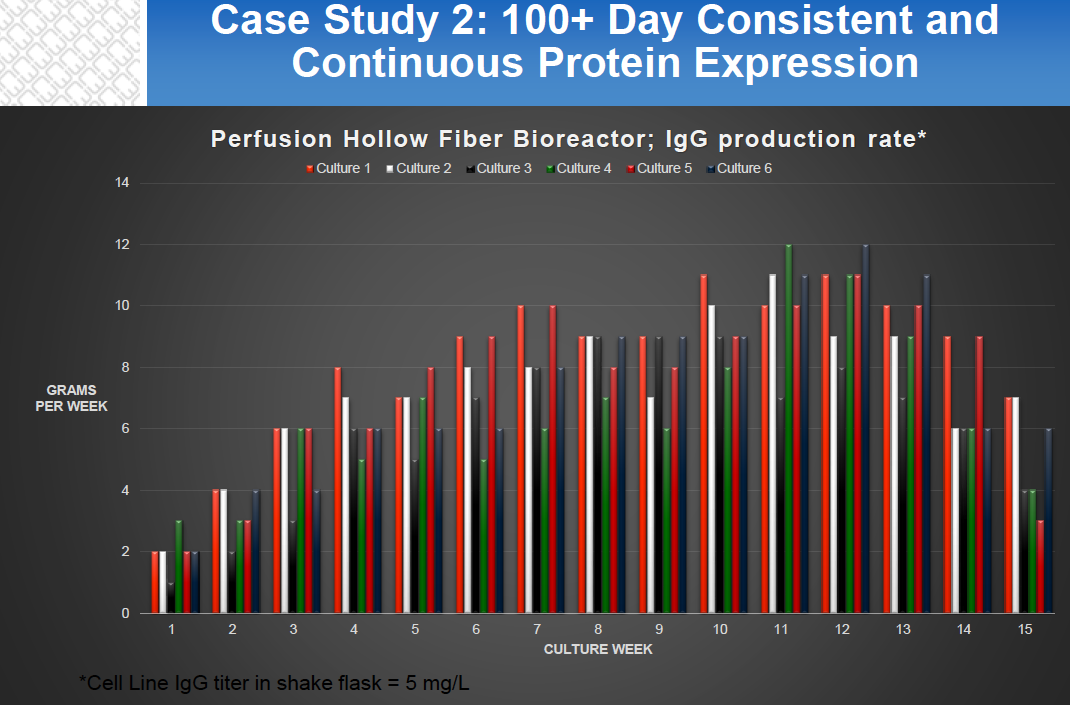
Figure 2:
Stirred Tank Bioreactors Reactors
Stirred tank bioreactors are most common throughout the biomanufacturing industry and have been used successfully in the manufacture of most biopharmaceuticals. They are a popular choice for conversion to perfusion because there is already a great deal of familiarity in using them for fed-batch, they are simple to operate, and there is much known about them. In order to convert them to a perfusion continuous process, they must be used with devices that permit them to be run in continuous mode. Necessary equipment would include filtration, centrifugation, or acoustic wave separation to maintain the cells while allowing the media and product to be removed.
Rocking Bioreactors
With rocking bioreactors, like GE Healthcare’s WAVE bioreactor, disposable bags are used as the bioreactor and the bags sit on a platform with rocks back and forth to stir the culture and enable oxygen transfer. For perfusion culture, these bags can come with a floating filter that provides the necessary filtration and the rocking motion prevents the filter from becoming clogged. To see a rocking bioreactor in perfusion mode, please see this video.
Fixed or Packed Bed Bioreactors
A fixed-bed or packed-bed bioreactor, such as the iCELLis® bioreactor from Pall offers a 3-D scaffold structure using a specific material such as nonwoven microfibers, a biopolymer or a hydrogel. The cells are entrapped in a fixed bed, providing an efficient growth environment for the cells. One advantage of a fixed-bed bioreactor is that they have very low shear stress due to the immobilization of cells.
Conclusion
In conclusion perfusion culture can offer several benefits that may help those in process development and manufacturing address key issues. The decision whether this technology is beneficial to specific products or manufacturing platforms is application dependent. While the benefits may be clear in some areas, there are still some questions that need to be address by companies’ looking to adopt this technology. Issues like what constitutes a batch, operation validation, in-process testing and how continuous upstream connects to the rest of the manufacturing platform needs to be carefully considered.
For more coverage of BPI West 2016, please see:
BPI West 2016 – BioProcess International Conference – In case you missed it!