
De-risking early stage clinical trials – an upstream continuous production solution for biologics
In this mini-webinar, Scott Waniger, VP Bioprocessing, Cell Culture Company, presents a cost effective, early stage clinical trial production process. This process utilizes a continuous upstream manufacturing approach, which reduces production time, lowers cost and simplifies scale-up.
To begin the webinar, Mr. Waniger describes current challenges in commercial drug manufacturing. With one of the most pressing, being the overall rise in aggregate costs. He explains that the overall cost of commercializing a drug, based on last year’s data is $2.6 billion dollars. This represents a 3 fold increase over just the past 12 years. In addition, these candidates have a high failure rate with only 1 in 10 Phase I drug candidates (1 in 20 for oncology drugs) reaching approval. These high costs coupled with high failure rates represents a huge financial risk for biopharmaceutical companies. As such, decreasing costs associated with clinical trials has been identified as a top priority for many pharmaceutical companies.
Understandably this risk has also caused several biopharma companies to be reluctant about committing significant resources to manufacturing until drug candidates have progressed in clinical trials and commercialization seems likely. Thus, companies are looking for ways to affordably manufacturing their early stage clinical trial material without significant capital investment.
Where can costs be cut?
Mr. Waniger continues the presentation with a discussion of areas where biomanufacturing costs can be reduced. He explains that while large-scale batch or fed-batch stirred tank bioreactors are very cost effective at making hundreds of kilograms of protein, they are not well suited for the sub-kilogram quantities needed for toxicology, animal studies, ex vivo, and early phase clinical trials. In addition, they are not often a good fit for difficult to express proteins. Scott describes how the Cell Culture Company (C3) uses their AcuSyst® perfusion bioreactor system for CDMO manufacturing, and how it is well suited for cost effective, sub-kilogram manufacturing batches. Providing the perfect capacity for most early clinical trial material manufacturing needs.
Key features of the technology:
No seed train, no clarification step required prior to purification
Scott explains that AcuSyst’s hollow fiber perfusion technology requires virtually no seed train and no need to clarify and concentrate harvest. The membrane based bioreactor system allows for controlled delivery of nutrients to allow for scale up in the bioreactor space, while concentrating the protein of interest on one side of the membrane yet permitting 95%+ of the media volume to be removed that does not contain the protein of interest. This concentration and cell debris removal process, allows the final bioreactor product to be chromatography ready as it is pulled out of the cell mass continuously. Figure 1 shows the process steps that can be eliminated with the AcuSyst perfusion bioreactor system.
Figure 1:
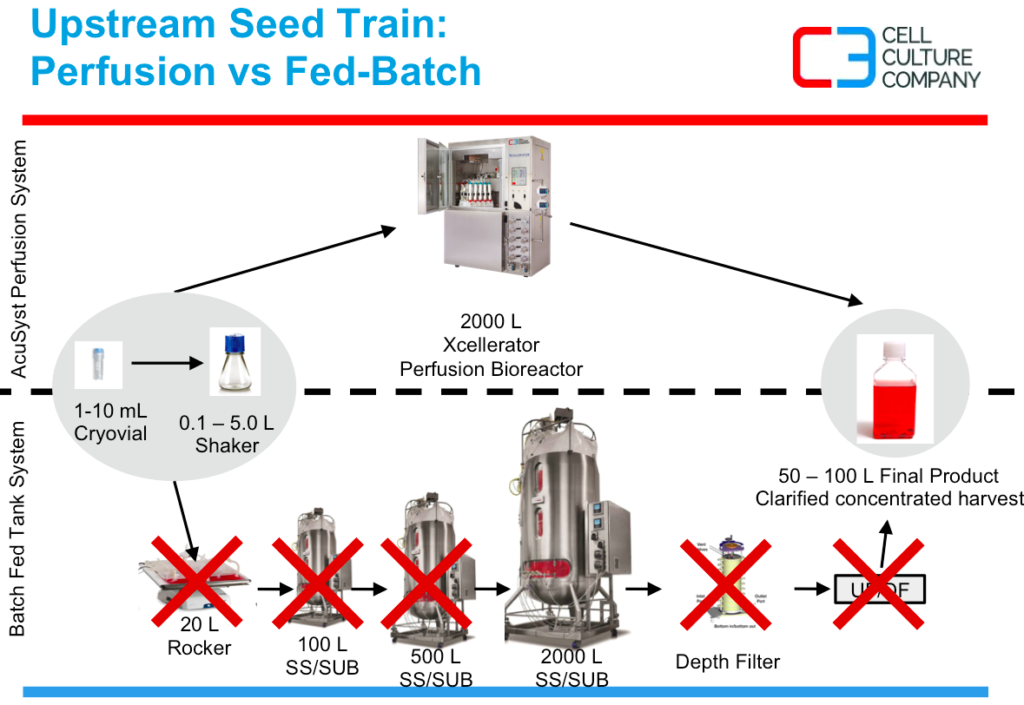
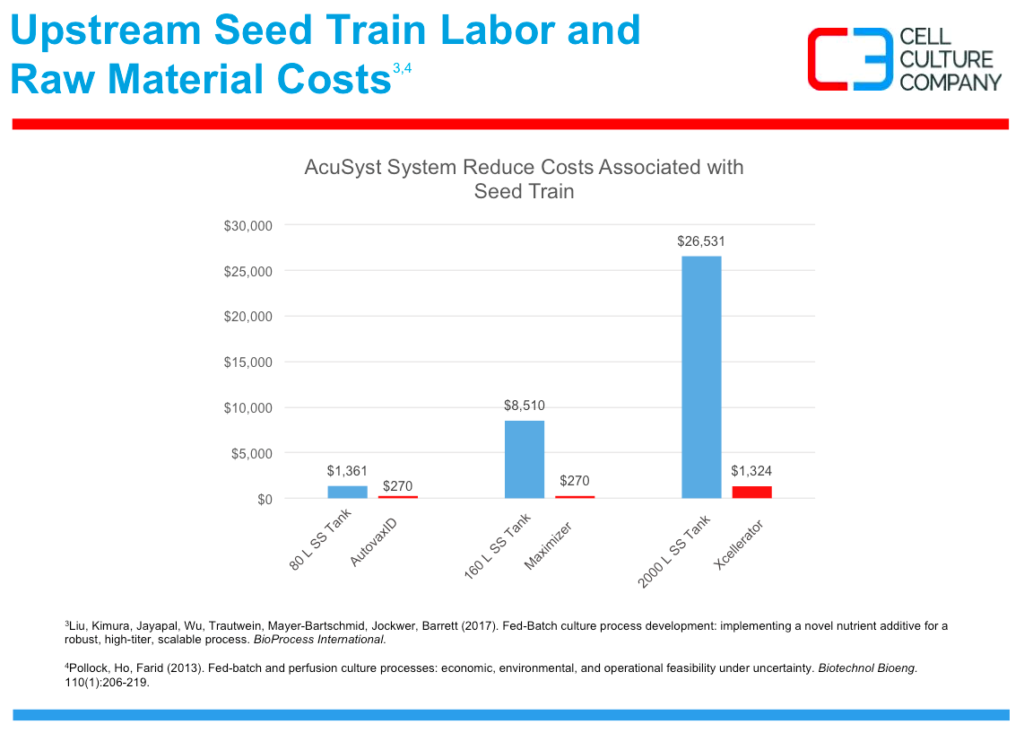
The lack of seed train also offers a significant reduction of upstream labor and raw material costs as seen in figure 2.
Figure 2:
Reduced Footprint and Utility Requirements Enable Flexible Facilities
Another benefit of the AcuSyst technology is the reduced footprint and utility requirements. The footprint of these bioreactors is very small considering output and come in a range of sizes to accommodate varying product demands. In addition to the space benefits, these systems are essentially plug and play and don’t require special facility configurations. Most systems can be operational with as little as a 100-240 VAC outlet, a CO2 source, and a network cable. This size advantage is important because it means that facilities don’t need a significant increase in space to increase production. Similarly, these bioreactors reduce utility costs and they are less labor intensive to operate. Thus requiring significantly less capital investment on the front end and lower operating costs to manufacture the same yield as fed-batch bioreactors.
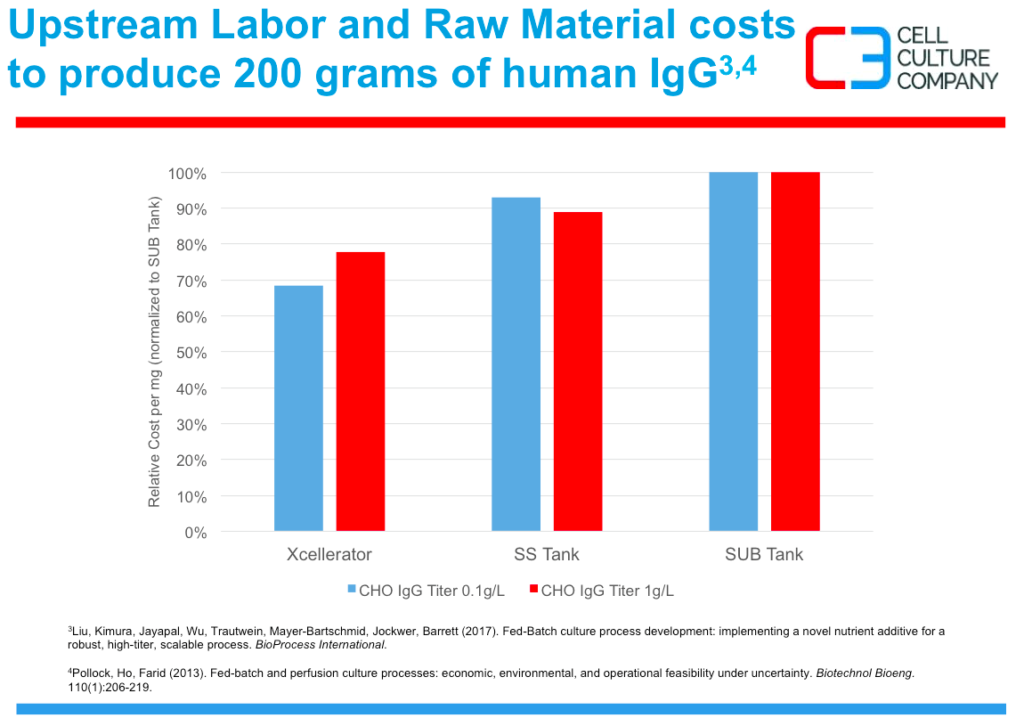
Figure 3 demonstrates the 20-40% reduction in cost when compared to tanks for labor and material
Figure 3:
Steady state metabolics and protein production
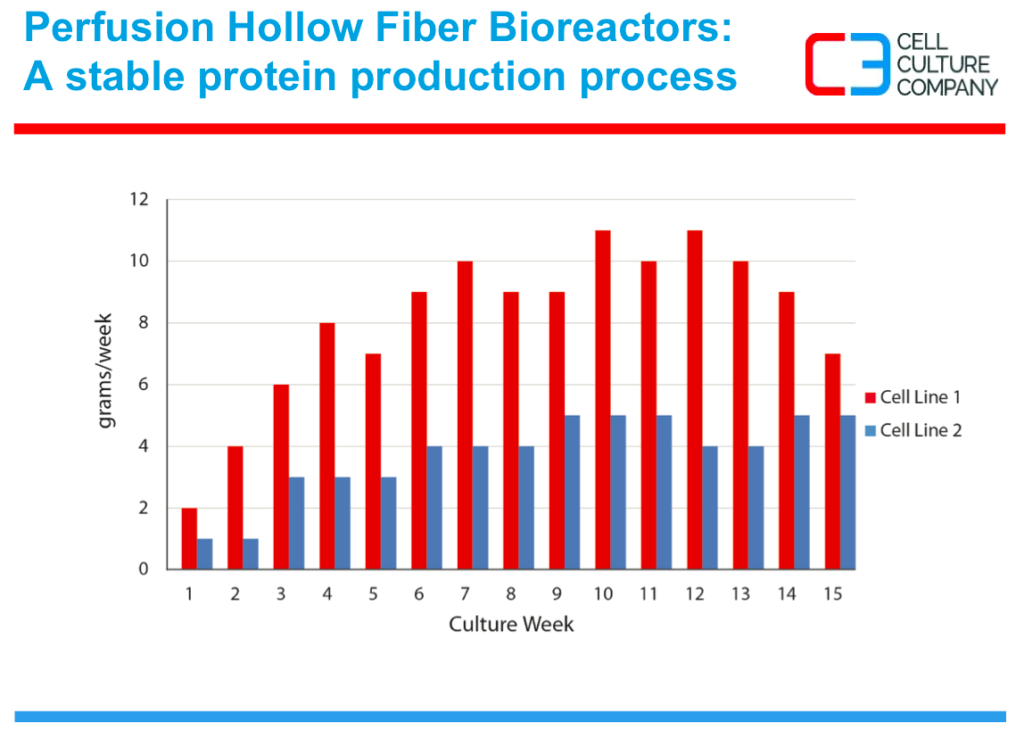
The third advantage with this technology is the ability to maintain steady state metabolics and protein production, thus protein manufacturing will not be determined by cell line stability, but by economic factors such as how much protein is required annually and the protein shelf life. These factors will help determine the length of the manufacturing process. As seen in figure 4, production is stable over many weeks.
Figure 4:
Scalability
Another advantage is that the AcuSyst technology is easily scalable. Additional product can be manufactured by simply extending run times or by adding additional cartridges. Once the process requirements for one cartridge are determined during process development, the process scales linearly, quickly, and predictably based on higher numbers of like cartridges in use. Figure 5 shows how protein production scales up linearly based on the number of cartridges.
Figure 5:
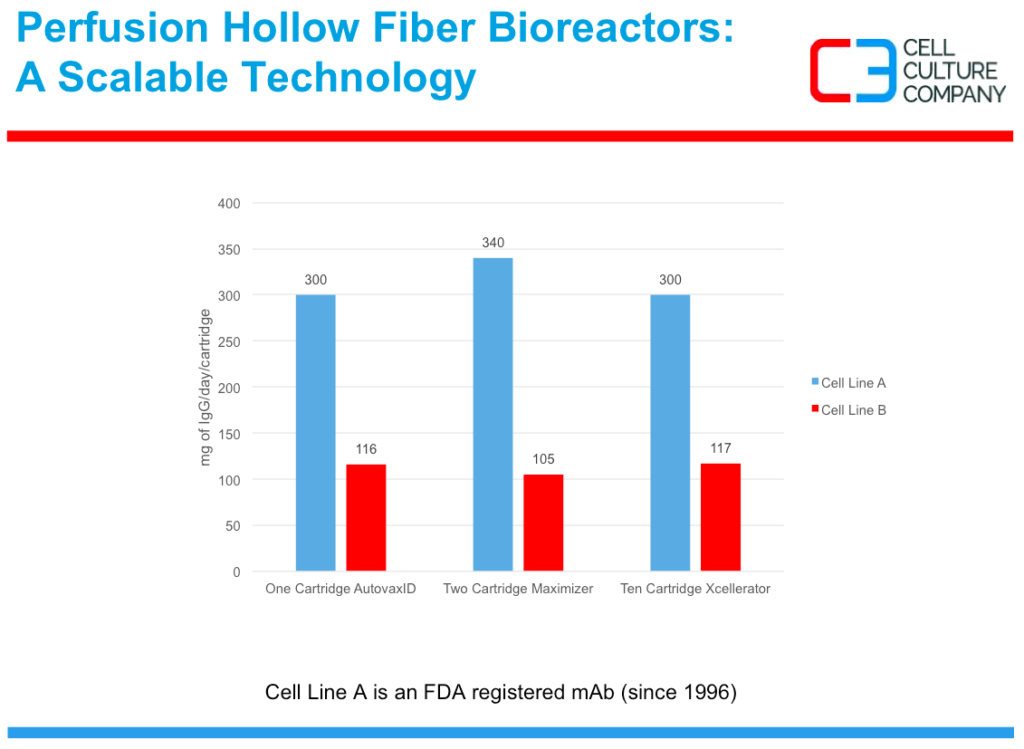
Difficult to express proteins
Next Mr. Waniger described that the more in-vivo like cell environment found in the AcuSyst hollow fiber system. The more in-vivo like environment supports higher cell densities, steady state metabolic activity and long lasting protein expression production. To demonstrate the enhanced ability to manufacture difficult to express proteins, Scott presented data on expression of two FC fusion proteins. By using this technology they were able to express more product using a smaller overall culture volume.
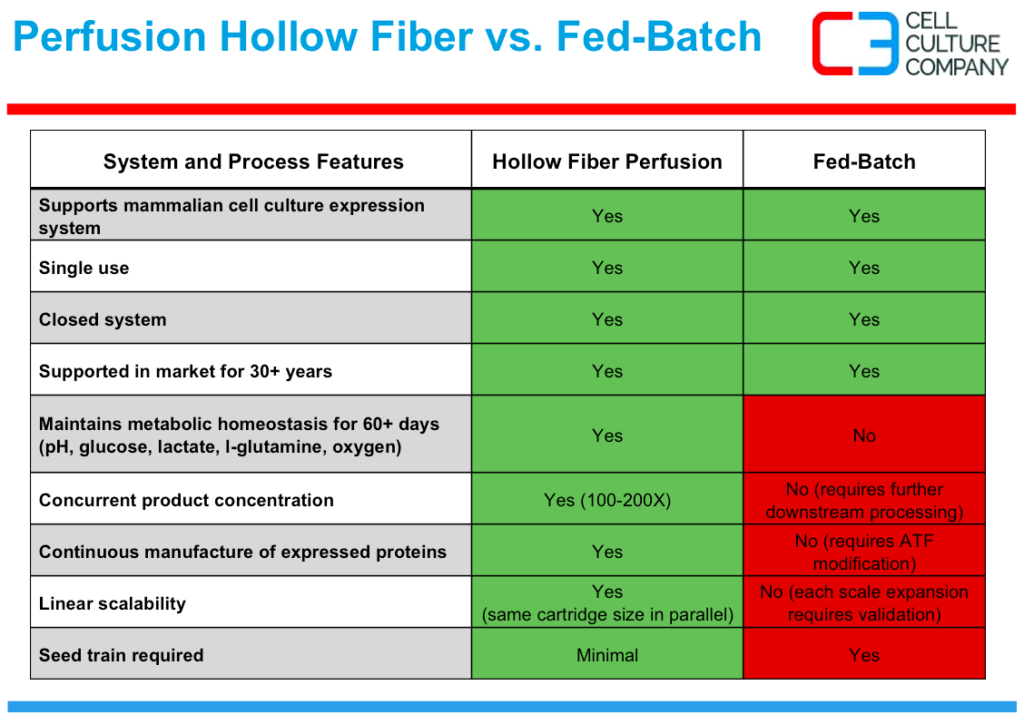
Perfusion Hollow Fiber vs. Fed Batch
Last Scott provides a comparison of fed batch stirred tank technology to perfusion hollow fiber bioreactors. The table shows the many similarities as seen in the first top four lines, as well as the differences, as noted in red, which provide the manufacturing cost reduction at sub-kilogram scale.
Summary
Mr. Waniger concluded the presentation by describing the long history of perfusion hollow fiber bioreactors with them being used in the market for 35+ years. They were originally patented to support mammalian cell culture in 1974, they are still in use today globally supporting manufacturing for in vitro diagnostics, clinical trial manufacturing and an FDA approved biologic.
For full data and more information, please watch Mr. Waniger’s presentation below:
About the Presenter:

Scott Waniger, VP Bioprocessing, Cell Culture Company
With 29+ years of extensive experience in mammalian cell culture manufacturing, Scott has been a key player in C3’s growth and development. His broad scope of knowledge in operations, management and regulatory needs compliments his hands-on experience in overseeing the expansion of 2,650 + unique cell lines, producing monoclonal antibodies, recombinant proteins, whole cell pellets, cellular extracts, and conditioned media from RUO to GMP applications. He has been fundamental in raising awareness and educating the life sciences industry on the benefits of perfusion based mammalian expression bioreactor systems. Scott has been with C3 since 1988.