Expansion of Mesenchymal Stem Cells in Blood-free, Chemically Defined Media
Last month’s ISCT conference held in Montreal, Canada featured many novel approaches for cell therapy research and manufacturing. One of the posters presented at the conference, “Inclusion of Recombinant Albumin and Transferrin Enables the Blood-Free Expansion of Mesenchymal Stem Cells in Chemically Defined Media,” highlighted the importance of blood-component free media for cell therapies and provided data on the use of a blood-free media in mesenchymal stem cell (MSC) culture.
Stem Cell Media
The inclusion of human plasma or plasma-derived proteins, such as albumin and transferrin, in stem cell culture media is common practice because the addition of these proteins have demonstrated benefits in multiple cell types. These proteins often serve as a substitute for fetal bovine serum (FBS) in the culture and permit a higher level of media definition than FBS. However these proteins, even when isolated from human serum, can still create problems associated with variability, stabilizing reagents, and the possibility of introducing contamination with adventitious agents.
Thus there has been much interest in creating cell culture media for therapeutic cell types that would be free of any animal or human blood sourced components. In this poster, authors’ present advances in recombinant protein expression that has permitted production of recombinant versions of serum albumin and transferrin. Non-mammalian expressed human serum albumin (Cellastim S) and recombinant human serum transferrin (Optiferrin) have been shown to be both biologically active as well as economically viable¹. Incorporating these proteins can produce media formulations that may simplify research and development with a higher degree of chemical definition as well as streamline the regulatory approval process for clinical products due the absence of blood-derived components. In the study, authors utilized Cellastim S and Optiferrin in the formulation of a blood-free media for mesenchymal stem cell (MSC) culture.
Poster Data Highlights
The blood-free media created using the recombinant albumin and transferrin was capable of efficiently expanding mesenchymal stem cells (MSC) isolated from multiple tissues when compared to xeno-free and serum- containing media. Additionally, MSCs cultured in this blood-free medium retained critical positive and negative MSC markers with minimal spontaneous differentiation after several subcultures.
Mesenchymal Stem Cell Culture Performance
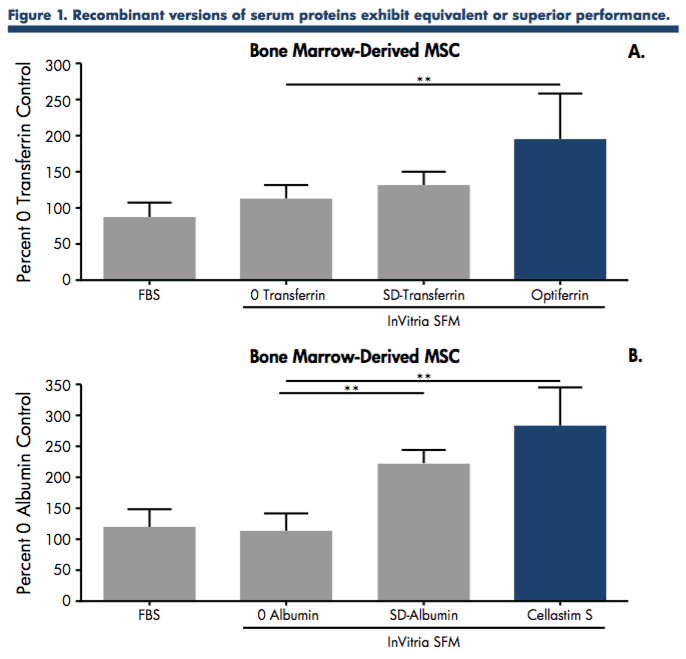
Authors’ compared the use of serum-derived albumin and transferrin to the recombinant versions, Cellastim S and Optiferrin, using a quantifiable functional performance assay in bone marrow-derived MSCs. This permitted a better understanding of how these proteins perform. The albumins and transferrins were formulated into a SFM specifically optimized for MSCs at a predetermined inclusion level and MSCs were allowed to expand in these different SFMs for 96 hrs using alphaMEM + 10% FBS as a standard control. Cells were harvested and counted and data was normalized against the 0 protein control (Figure 1).
The functional screen determined that the recombinant versions of albumin and transferrin exhibited equivalent or superior performance to that of the native serum derived versions in expanding low passage MSC isolated from human bone marrow. Both native and recombinant versions, however, exhibited improved performance from the 0 protein and FBS controls.
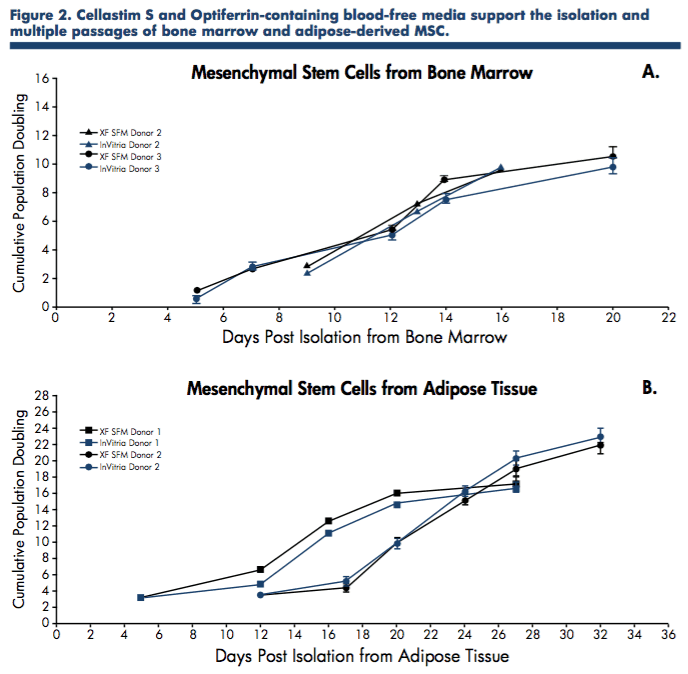
To further confirm these initial findings, mesenchymal stem cells derived from human bone marrow and adipose tissue were expanded in serum-free media containing the recombinant proteins, Cellastim S and Optiferrin, and compared to commercially available xeno-free serum- free media in the isolation and expansion of MSC from both tissue sources.
Again, the blood-free media containing Cellastim S and Optiferrin exhibited equivalent expansion capability to media containing human serum albumin and transferrin (Figure 2).
Mesenchymal Stem Cell Morphology and Phenotype
Cells expanded in Cellastim S and Optiferrin also appeared to be morphologically normal during expansion. Cells also exhibited normal phenotype of bone marrow and adipose derived MSC. (See figure 3 & 4 on poster for supporting data)
Please see poster for complete data and methods – Inclusion of Recombinant Albumin and Transferrin Enables the Blood-Free Expansion of Mesenchymal Stem Cells in Chemically Defined Media
Also, don’t miss the InVitria webinar on this topic:
Using Blood-Free Insulin, Transferrin, and Albumin Supplements to Reduce and Eliminate FBS in Research & Manufacturing
Thursday, June 28th 12-1pm MT
See How Blood-Free Supplements Add Consistency & Reduce Variability
It is well known that serum and other animal-derived components can create inconsistency in cell culture, leading to variability in cell growth, phenotype, and functional performance. Therefore, it is critical to both manufacturing and research applications to reduce or completely eliminate the use of these ingredients.
InVitria offers blood-free cell culture supplements that effectively reduce the amount of serum needed in routine cell culture by replacing the insulin, transferrin, and albumin found in serum with recombinant versions. These supplements, known as ITSE AF, ITS AF, and ITSE + A AF, also serve as a suitable foundation for fully serum and blood-free cell expansion.
References:
- Generating Recombinant Versions of Human Serum-Derived Proteins – Transferrin and Albumin, Cell Culture Dish
- Albumin in Cell Culture Media – An examination of quality and function, Cell Culture Dish
- Albumin Fatty Acid Profiles for cell culture media – Enabling Albumin Optimization for Cell Culture Media