
Fit for purpose, closed automated cell processing system provides improved cell therapy workflows for research through manufacturing
Cell and gene therapies are poised to provide a vast new arsenal of therapies to treat disease. Much like monoclonal antibody manufacturing in the early 1980’s, cell and gene therapy manufacturing is constantly evolving with improved fit-for-purpose technologies being developed to meet expanding industry need. Despite smaller volumes, cell and gene therapy manufacturing is more complex and labor intense than protein-based biologics. Cell and gene therapy processing requires multiple steps and procedures including cell separation, concentration, washing and others. For a successful cell therapy to be produced, it is important to be able to conduct these activities as simply, safely and efficiently as possible.
The Rotea System
A recent poster presented at American Society of Gene and Cell Therapy annual meeting 2021, Building New Cell Therapy Workflow Solutions by Leveraging the Flexibility of the Rotea System’s Closed Automated Cell Processing Technology, highlights a highly efficient, closed and automated system for cell processing. The Gibco™ CTS™ Rotea™ Counterflow Centrifugation System is a fit for purpose solution to enable safe and cost effective cell therapy workflows with scalable throughput and continuous processing of up to 20L .
Gentle Isolation
A key feature of the Rotea system is its gentle isolation for even the most sensitive cells. It employs counterflow centrifugation (CFC) that suspends cells in a fluidized bed by exerting a constant flow force opposite centrifugal forces to provide capabilities otherwise impossible in traditional centrifuges. Fluid suspended cells are concentrated without forming a pellet in the cone and then are washed quickly and efficiently with high recovery rates. Due to the reduced cell density, dead cells can be removed to optimize viability before delivery. By fine-tuning centrifugal speed and flow rate, cell types of different sizes can be effectively separated.
Closed System
Closed systems are particularly desirable in clinical and commercial cell therapy operations as they offer the highest containment and product safety protection. The ability for the Rotea system to function as a closed instrument provides a cost benefit as well, because one equipment capital expenditure can be utilized from research through commercial manufacturing.
In addition, using the same equipment from research through process development and manufacturing can cut process development times and delays by eliminating the need to learn and optimize new systems. Closed systems also reduced the requirements and associated costs for clean room space as they can operate in a Class C environment, thus reducing the need for Class B space that is more costly to set up and operate. It also supports a flexible facility by permitting multiple batches to be processed in a shared clean room.
Versatile
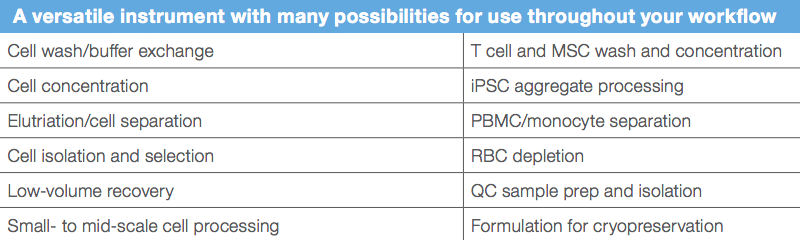
The Rotea system is highly versatile enabling several cell therapy workflow operations. It operates using programmable software that allows users to create and optimize a wide range of protocols for cell separation, washing and concentration. This enables users to conduct many operations in a single system (Table 1), thus reducing overall footprint and valuable facility space. Using a proprietary technology, low output volumes can deliver as little as 5 mL of concentrate. A universal kit is available as a consumable for different cell types and applications. Another unique feature, the Gibco™ CellCam™ is a color camera that visualizes cells live in a fluidized bed, enabling CFC parameter optimization. This allows users to easily fine tune protocols for each application.
High Quality Results
Cell therapies rely on cell isolation, wash and concentration. Increasing the productivity of these processes permits quicker turnaround time for patients and also can reduce the cost of cell therapy manufacturing. Automation is a key component of improving efficiency. The Rotea system is designed to efficiently isolate peripheral blood mononuclear cells (PBMC) from leukopaks and wash and concentrate T cells (Figure 1), two vital steps in many cell therapy workflows.
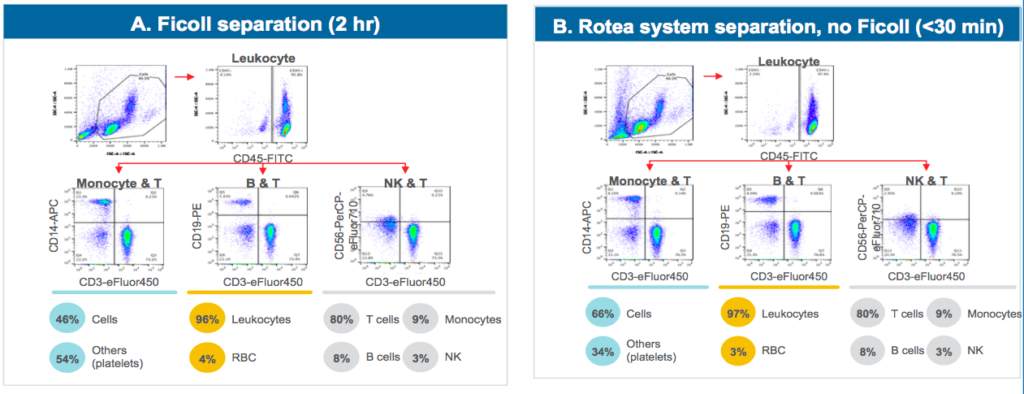
High Efficiency
A common method for cell separation is to use a density gradient such as Ficoll. When compared to Ficoll separation, T cells processed using the Rotea system exhibited similar quality through expansion with thorough platelet and red blood cell (RBC) depletion (Figure 2). The primary difference is that the Rotea system separation is much more efficient, requiring less than 30 minutes while maintaining a closed system as compared with the two hour open process for the Ficoll separation.
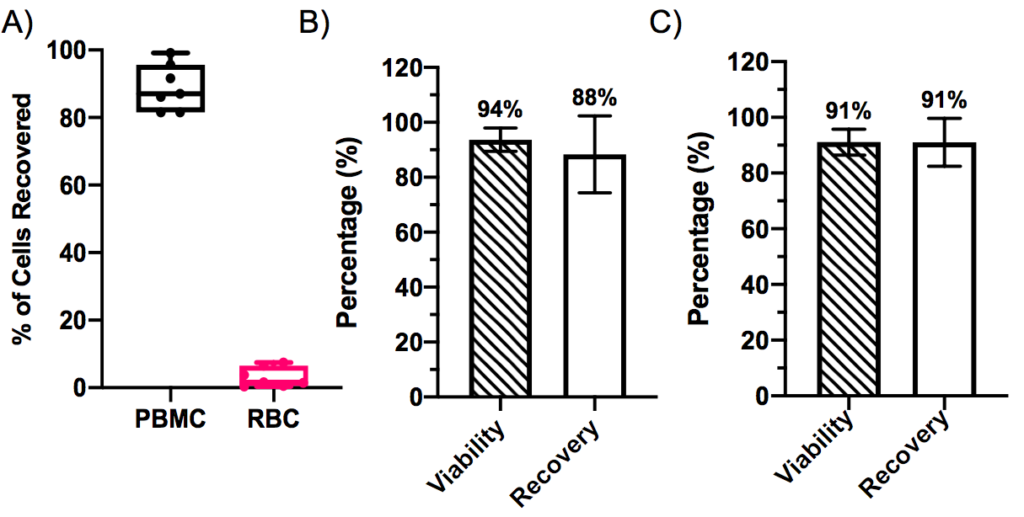
High Viability and Recovery
The Rotea system can achieve approximately 90% PBMC recovery from a leukopak bag in combination with a red blood cell lysis buffer across multiple donors (n=7) (Figure 3). For frozen leukopaks, the average viability and recovery exceeded 90%. Thus, demonstrating similar efficacy with fresh and frozen leukopaks. In this experiment, the Rotea system was also used to remove cryopreservation medium and wash into growth medium. Similar viability and recovery results were demonstrated for natural killer (NK) cells (data provided in poster).
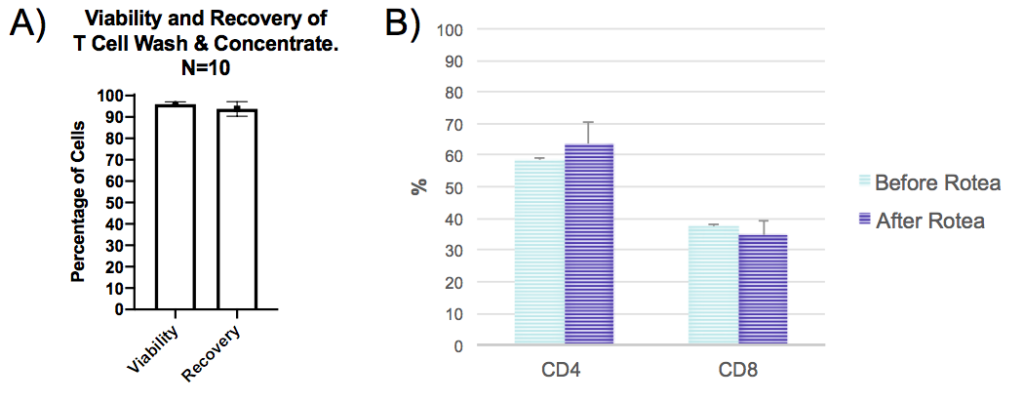
High Quality
A key element of successful cell therapies is the quality of the cells. In Figure 4, T cell recovery and viability over 90% was demonstrated for ten runs of wash and concentrate. A comparison was made between the composition of CD4+ and CD8+ cells before and after wash and concentrate. The populations were consistent between the two analyses, thereby demonstrating that cells were unaffected by Rotea system processing.
Summary
The Rotea system provides an efficient, flexible, and cost-effective solution for cell therapy processing. Data demonstrates high recovery, viability and quality of both T cells and NK cells after processing by the Rotea system. Lastly, its closed single-use kit permits smooth transition from research through commercial manufacturing while minimizing risk of contamination.