
Improved, High Quality Poloxamer 188 Produces Consistent Performance in Cell Culture
Hydrodynamic stress in bioreactors and specifically shear caused by sparging can present a challenging issue in commercial biopharmaceutical manufacturing. A solution was found in the use of Poloxamer 188, a surface-active, non-ionic polymer that when added to cell culture media acted as a shear protectant. Poloxamer 188 became a standard ingredient in cell culture media for commercial production.
However, as cell culture technology improved including process intensification, which increased cell densities and productivities in fed-batch and perfusion modes, issues with poloxamer were reported. Issues included unexpected loss of cell density and viability in biopharmaceutical manufacturing. This loss could be traced back to lot-to-lot variation in the Poloxamer 188. This variation was a major problem and was the subject of several investigations to understand the source of the variation.
To address this issue, MilliporeSigma developed proprietary analytical and biological methods to identify the critical properties of Poloxamer 188. These methods were based on a reference library of over 100 blinded customer and supplier samples.
Improved Poloxamer 188
Using this knowledge, MilliporeSigma recently launched Poloxamer 188 EMPROVE® EXPERT cell culture optimized, which was developed to provide reliable quality, consistency, and shear stress protection for large scale cell culture processes.
Benefits include:
- Consistent quality — With in-house developed methods to predict the performance and ensure lot-to-lot consistency.
- Proven functionality — Shear protection tested and implemented as a release criterion.
- Superior performance — Superior product performance across different suppliers, batches and quality grades.
- Reliable supply — Large manufacturing capacities ensure reliable supply.
- Full transparency — Full GMP documentation with EMPROVE®
Ensuring reproducible performance at larger production scales
MilliporeSigma selected two methods to demonstrate reproducible performance at larger production scales – a biological as well as analytical assay.
Biological Assay
A rapid cell-based shear protection assay was developed as a cell-based method to assess and classify the shear protective effect. A cell test has been implemented for standard product release.
Analytical Assay
In addition to the biological assay, analytical methods were developed to look at the molecular weight distribution of different Poloxamer 188 lots. Based on these attributes, MilliporeSigma developed an analytical method to reliably distinguish between good and poor performing lots.
In figures 1 and 2, you can see the results of an analytical assay and a biological assay used to ensure good performing lots.
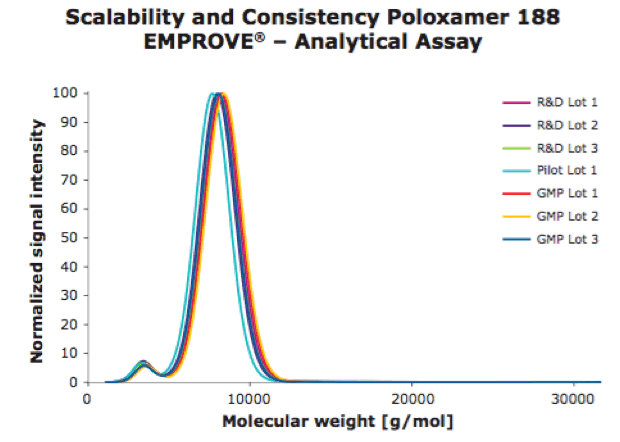
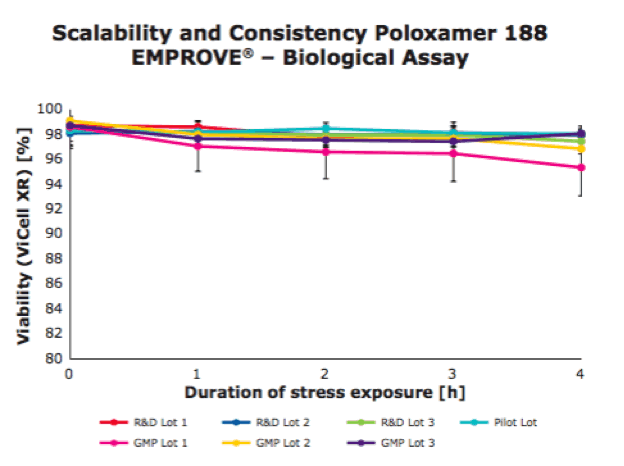
For more information, please see – Poloxamer 188 EMPROVE® EXPERT
“Ask the Expert – Managing Shear Stress in Biomanufacturing with the shear protectant Poloxamer 188 – A Discussion,” Cell Culture Dish
During this Ask the Expert session, we had questions that covered topics including factors that contribute to shear, analyzing and modeling shear stress in a bioreactor, and how to use a shear protectant to protect against shear. In addition, we had several questions related to Poloxamer 188 implementation, best practices, cell lines, qualification and media concentration level…”