
Innovative Solutions for mRNA Vaccine Manufacturing: A Practical Guide Using PolyPlus LipidBrick® and Services
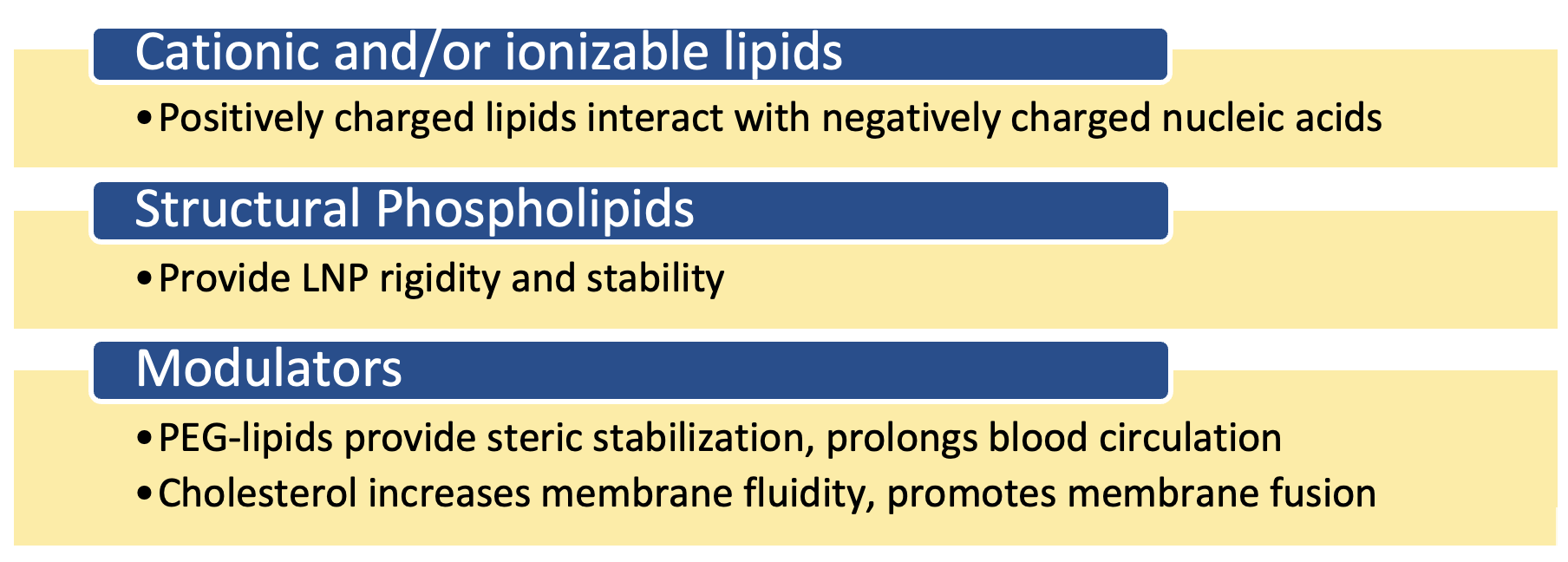
Lipid nanoparticles (LNPs) play a crucial role in the development and manufacturing of DNA and RNA therapeutics including the Pfizer-BioNTech and Moderna messenger RNA (mRNA)-based COVID-19 vaccines. LNPs serve as non-viral delivery vehicles that encapsulate and transport mRNA into target cells, where the mRNA can be released and translated by cellular machinery to produce the protein of interest that will elicit an immune response. LNPs are composed of different lipid species including cationic and/or ionizable lipids, structural phospholipids, PEG-lipids, and cholesterol.
The mRNA vaccine manufacturing process uses microfluidic systems, where the synthesized mRNA and the lipid mixtures are homogenized within a laminar flow to induce mRNA-LNP self-assembly. The resulting LNP drug product can then be processed further, purified, and analyzed downstream. A deep understanding of the formulation steps and microfluidic mixing are critical to scalable, reproducible, and high-quality LNPs production.
Polyplus’ new infographic outlines the key steps for successful mRNA vaccine production, which encompasses mRNA manufacturing using their plasmid engineering services and LNP formulation process with their novel LipidBrick® IM21.7c cationic lipids optimized for mRNA delivery. Of the LNP lipid components, the cationic lipids play a crucial role in modulating the LNP properties including LNP stability, tissue selectivity, cellular uptake, immunogenicity, and more. The intrinsic properties of the LipidBrick IM21.7c imparts an overall positive charge to the formulated LNP to enable a wider in vivo biodistribution and reduce liver accumulation, which can improve drug potency, safety, tissue targeting, and formulation stability.
As an innovator in the delivery field, Polyplus’ cationic lipids have also been successfully used in both in vitro and in vivo proof-of-concept studies to demonstrate their potential for mRNA-LNPs formulation, providing a simpler and more cost-effective manufacturing route for drug developers to meet the growing demand of the mRNA therapeutics market while also broadening the scope of LNP usage to other biopharma applications.
To learn more about how LipidBrick® IM21.7c can opens new possibilities to improve your LNP formulation, please visit: https://www.polyplus-transfection.com/products/lipidbrick/