
Keys to successful single-use bioreactor scale up – from small scale to pilot scale
Successful monoclonal antibody (mAb) manufacturing relies on good process development and reliable bioreactor scale up. Initial process development work is typically conducted at small scale, then moved to bench scale and lastly to pilot scale before moving into manufacturing. One of the biggest challenges is scaling up an optimized process from process development to manufacturing scale. Just a small change in the size of the bioreactor can have a cascade effect on many other culture conditions.
While small-scale models are powerful tools in upstream process development, there are often differences with small-scale data and what is seen at bench-scale or at larger production. In order to ensure good scalability it is important to consider key aspects of each subsequently larger vessel in scale up.
Keys to Successful Bioreactor Scale Up
A recent poster, Cell Culture Scale-Up in BioBLU® Rigid-Wall, Single- Use Bioreactors, describes the steps necessary to ensure successful scale up using single-use vessels. It is well known that single-use bioreactors can provide advantages over non-disposable bioreactors in many instances. Single-use bioreactors provide a faster turnaround time through the elimination of cleaning, assembling and autoclaving operations. They can also reduce water use and validation requirements.
In the poster, authors used BioBLU Single-Use Vessels of different sizes (maximum working volumes of 0.25 L, 3.75 L, and 40 L) that are of geometrically similar stirred-tank design to scale up a mAb CHO process from small to pilot scale production.
Bioreactor Scale Up Considerations
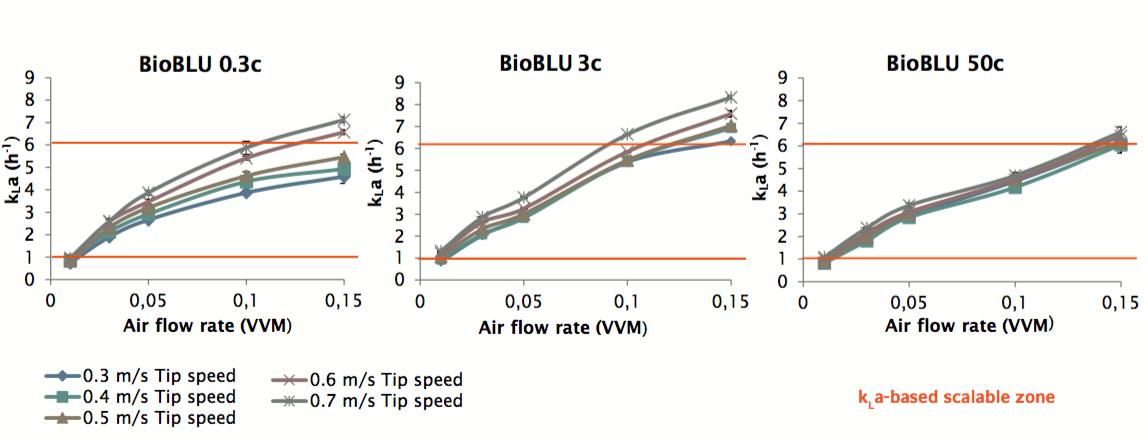
First the authors highlight the importance of looking at each vessel in the scale up plan and evaluating the specifications. Ideally, each vessel should be geometrically and proportionally similar. For the BioBLU vessels, authors identified a wide scalable tip speed zone (0.3 – 0.7 m/s) and an overlapping range of kLa values for that would accommodate most mammalian cell culture requirements. Authors point out that maintaining a constant kLa between vessels of different sizes is a frequently used strategy for cell culture scale-up. It is important to select equipment with similar kLa capabilities that provide sufficient overlapping, thus ensuring small-scale success can be replicated at larger scales. Figure 2 shows the BioBLU kLa values for each volume size.
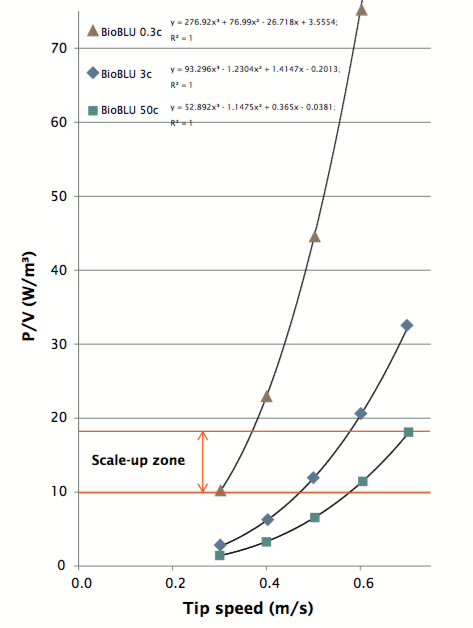
Next, the authors determined power numbers of the BioBLU bioreactors. The dimensionless power number Np was determined for different BioBLU Single-Use Vessels at different stirrer speeds. The purpose of determining Np is to calculate impeller power consumption per liquid volume (P/V, W/m3 ).
Authors explain that maintaining constant P/V (impeller power consumption per liquid volume) between vessels is another widely accepted strategy for scale-up. In this study, the CHO culture was scaled up with a constant P/V of10.9 W/m3. This lower P/V value within the scale-up zone was chosen to reduce tip speed and shearing. Figure 3 shows the P/V values for different volume vessels.
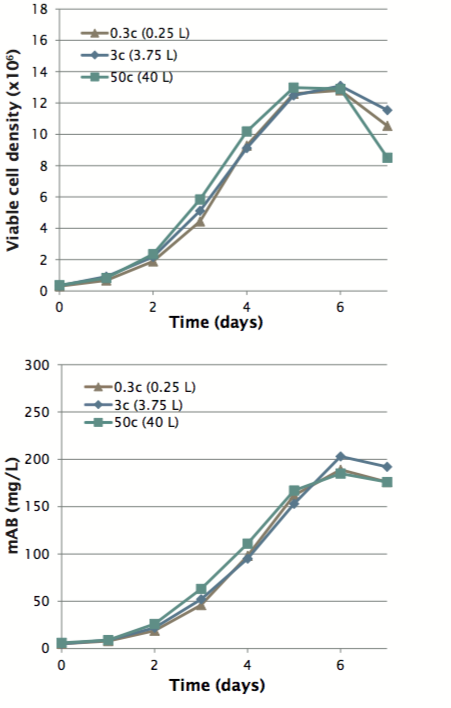
Based on these data authors scaled up a process for production of monoclonal antibodies (mAb) in CHO cells from 0.25 L to 3.75 L to 40 L by keeping constant P/V values among the differently sized vessels. Similar cell growth curves and mAb production profiles were achieved at all three scales (Figure 4).
Conclusions
The authors provide a very informative strategy for scaling up a CHO process from small to pilot scale while maintaining excellent consistency in results. The vessels are all geometrically and proportionally similar, and there is an overlapping range of P/V values providing excellent scalability.
For more information about the study and bioreactor scale up, please see full size version of poster above.
For more information about BioBLU Single-Use Vessels, please see www.eppendorf.com/BioBLUc.