Media Optimization Strategies for Modifying Monoclonal Antibody Quality Attributes
Cell culture media optimization continues to play a critical role in both improving titers and providing a mechanism for modifying monoclonal antibody (mAb) quality attributes. Furthermore supplements and trace contaminants in the media can have dramatic effects, both positive and negative, on productivity, growth and protein quality. This highlights the importance of identifying media components that drive both productivity and desired quality profiles to select the best media formulation for each monoclonal antibody product.
I recently viewed a very insightful webinar on this topic. In the webinar, “Impact of Media and Supplements on Monoclonal Antibody Quality Attributes, Dr. Neelanjan Sengupta, Scientist III, Thermo Fisher Scientific describes the ways in which media components can be leveraged to achieve desired monoclonal antibody quality attributes and strategies for creating media formulations that provide good productivity as well as the desired quality profile.
Dr. Sengupta began the webinar by providing background information on monoclonal antibody quality including the fact that specific quality attributes are key for proper antibody function both to ensure that immunogenicity is not triggered and that efficacy is achieved.
Protein Quality Attributes are Critical for Monoclonal Antibody Function
While there are multiple attributes of monoclonal antibody quality, Dr. Sengupta focused on three primary attributes; glycosylation patterns, charge variants and protein aggregation. It is important to understand these critical quality attributes for both originator and biosimilar drugs. He explained that with originator drugs, understanding critical quality attributes ensures mAb quality and required protein function. For biosimilar drugs, the biosimilar approval process requires proof that the candidate shows no analytically or clinically significant differences and demonstrates similar pharmacokinetics as the originator molecule.
Bioproduction factors that impact mAb quality
Next Dr. Sengupta discussed the three primary bioproduction factors that impact mAb quality, these include: the cell line itself, process parameters and cell culture medium/supplements. For the purposes of this presentation, he focused on leveraging media formulation to adjust quality, for a desired outcome.
Impact of media formulation on protein quality attributes
Media components can have positive and negative impacts on protein quality attributes. Dr. Sengupta explained that certain trace metals and amino acids impact glycosylation in predictable ways. Media formulation can directly affect quality as certain components have been shown to increase or decrease certain quality attributes.
Media can also impact quality indirectly. For instance, media that increases productivity and viable cell density (VCD) can indirectly impact quality as high specific production rates have been associated with suboptimal glycosylation. Also cell culture conditions including excess ammonia and/or lactic acid production can negatively influence both protein production and protein quality.
While there are several quality attributes impacted by the production process, Dr. Sengupta focused on three primary attributes in his presentation – glycosylation, charge variance, and aggregation.
Glycosylation
Dr. Sengupta then provided an overview of the glycosylation process. Glycosylation refers to the attachment of oligosaccharides at Asn297 on the heavy chain of the mAb. This is the result of posttranslational mechanisms of the cell and involves complex enzymatic pathways. N-linked glycosylation affects mAb conformation, solubility, circulatory half-life, and functions such as antibody-dependent-cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). The N-glycan species distribution indicates the unique functional characteristics of a mAb and will be specific to the drug molecule. More specifically, galactosylation and fucosylation can affect ADCC and CDC and sialic acid content can impact anti-inflammatory activity and serum clearance efficacy. Low-mannose species are considered desirable in general, due to low immunogicity.
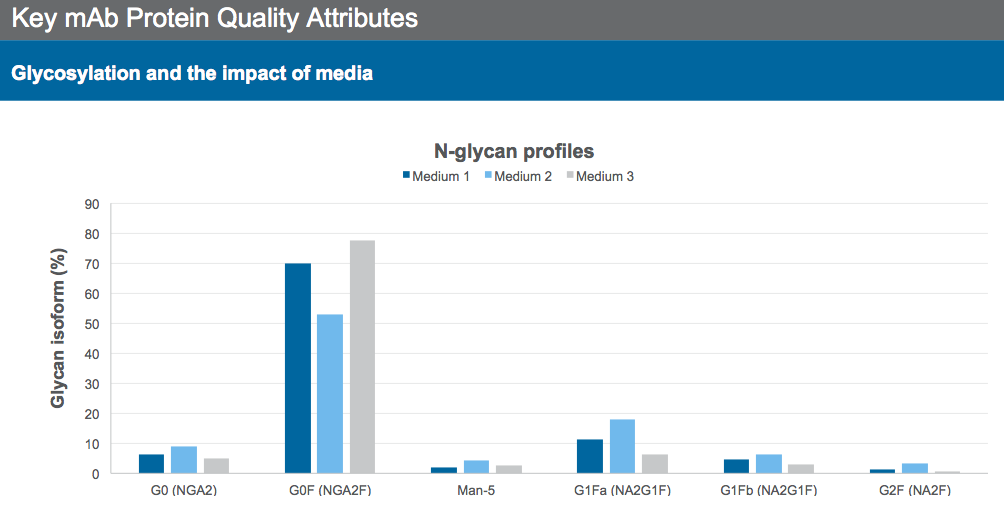
Growth, production and N-glycan profiles of mAbs vary depending on the medium used. To demonstrate the role of different base media in modulating the N-glycan profile, Dr. Sengupta presented data for the N-glycan profile of three different media, each generated a different profile (Figure 1), using the same mAb producing CHO cell line and process.
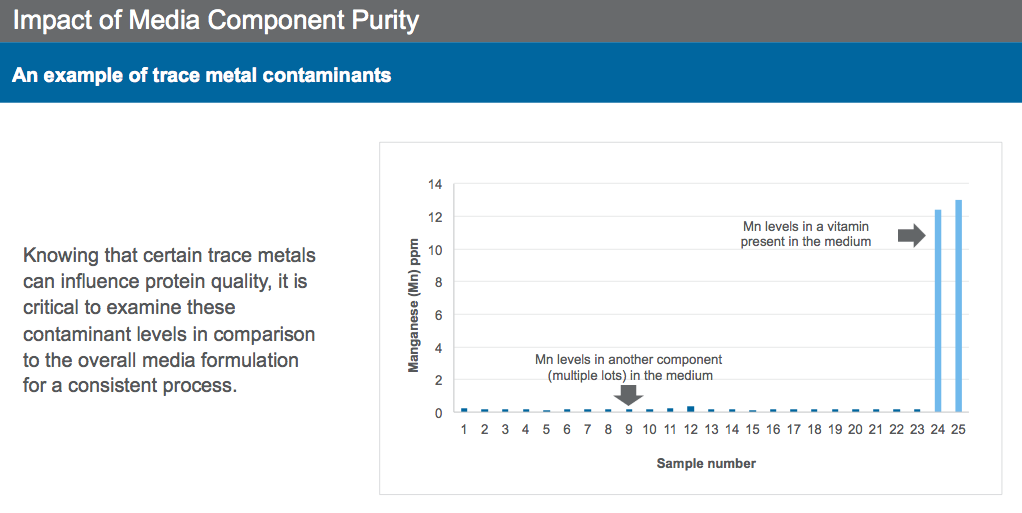
Furthermore, media purity has a big impact on N-glycan profiles. For example, Dr. Sengupta shared two references where trace metals have been shown to impact culture performance and protein quality. Knowing that certain trace metals can influence protein quality makes monitoring of contaminant levels in media and supplements critical in ensuring a consistent production process. Manganese, copper and iron have all been shown to effect N-glycan profiles.
In one presentation example, the manganese level in a commonly used vitamin for cell culture resulted in unintended shifts in product quality attributes (Figure 2).
Beyond understanding the impact of how various components and impurities impact N-glycan profile unintentionally, it is possible to optimize media to intentionally adjust N-glycan profiles to fit required quality profiles.
Looking at galactosylation for example, the addition of chemically defined (CD) protein quality supplements to an existing base medium produced an increase in galactosylation, while maintaining mAb production and cell growth (Figure 3).
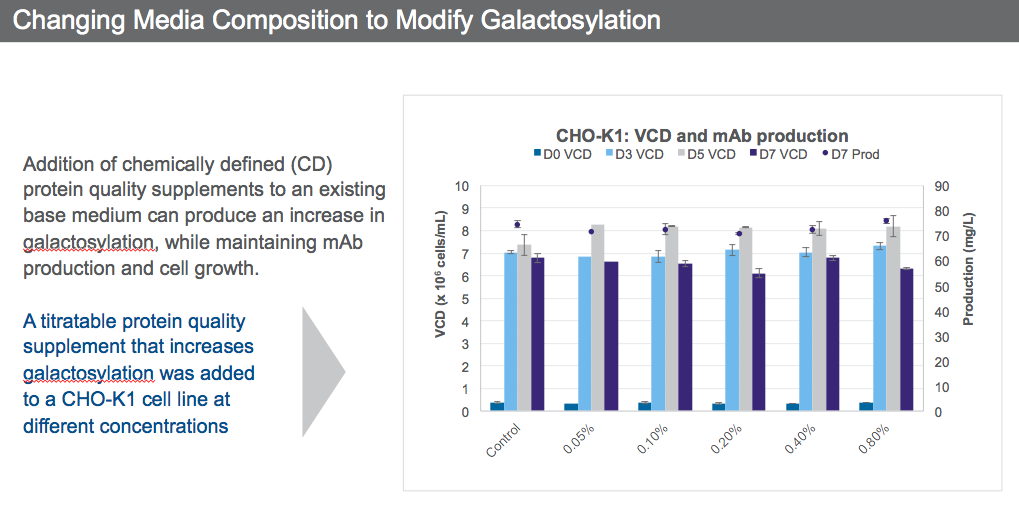
A titratable protein quality supplement that increases galactosylation was added to a CHO K-1 cell line at different concentrations (Figure 4).
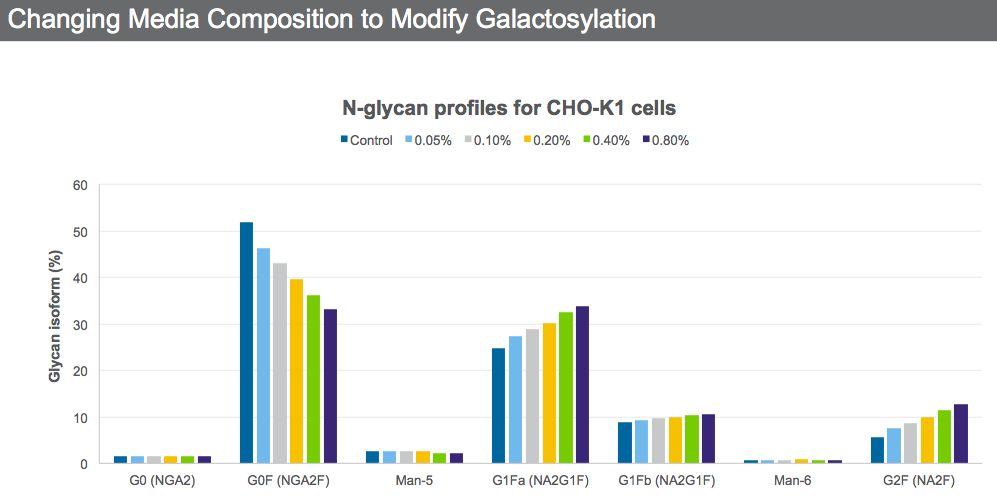
It is possible through media optimization to obtain the desired N-glycan profile while still maintaining VCD and productivity by selecting the supplement concentration that will give the desired quality attributes.
Charge Variants
Next Dr. Sengupta discussed charge variants and how they are a mixture of antibody molecules consisting of acidic (lower isoelectric point), main peak and basic (higher isoelectric point) variants. Different charges on mAbs result from events during cell culture production including sialylation, deamidation, oxidation, isomerization, C-terminal Lys clipping, and incomplete disulfide bonds.
Charge variants can affect the performance of mAbs in the following ways:
- Acidic variant – deamidation can potentially impact binding affinity depending on the location on the mAb chain.
- Basic variant – a Fab with unpaired disulfide bonds is only about 28% as potent as the Fab containing the fully formed disulfide bond.
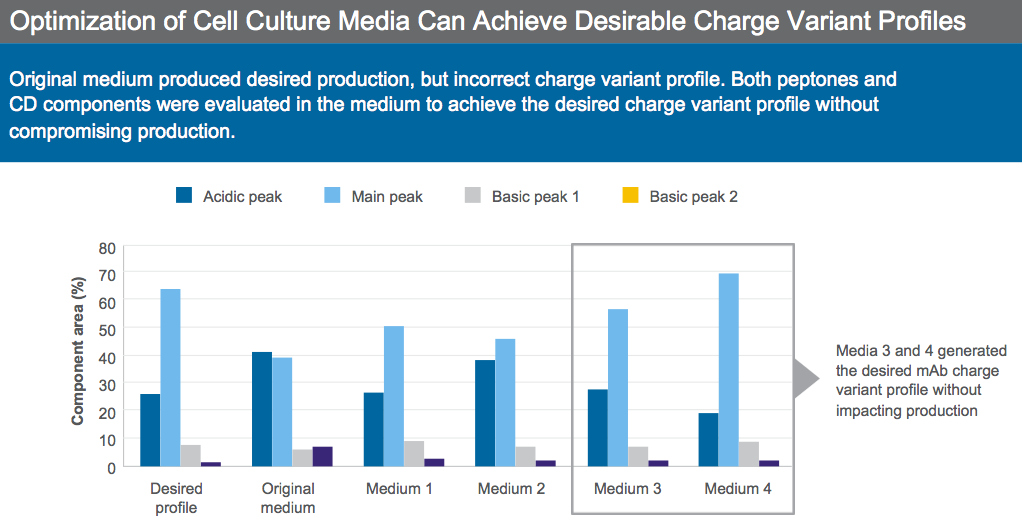
From the literature, we know that media components can impact charge variants, in particular copper concentration can affect charge variant levels. Dr. Sengupta presented an example of a biosimilar process where the original media provided good productivity, but the wrong charge variant profile (Figure 5).
Peptones and CD components were evaluated in the media to achieve desired charge variant profile. After optimization, two media candidates provided the desired charge variant while maintaining productivity and were selected for further development (Figure 5).
Aggregation
For the third attribute, protein aggregation, Dr. Sengupta explained that it is the process where mAbs assemble into stable complexes of two or more molecules. A monoclonal antibody’s primary sequence and structure can influence aggregation. Media, the purification process, and product formulation can also lead to protein aggregation as these processes impact protein folding and stability. Protein aggregation can decrease bioavailablity and efficacy of the final product and may elicit undesired immune responses to the drug.
Dr. Sengupta explained that in the literature there are several examples of how media components impact aggregation. Certain sugar molecules and other osmolytes may reduce protein aggregation. Cysteine, iron and copper can increase protein aggregation.
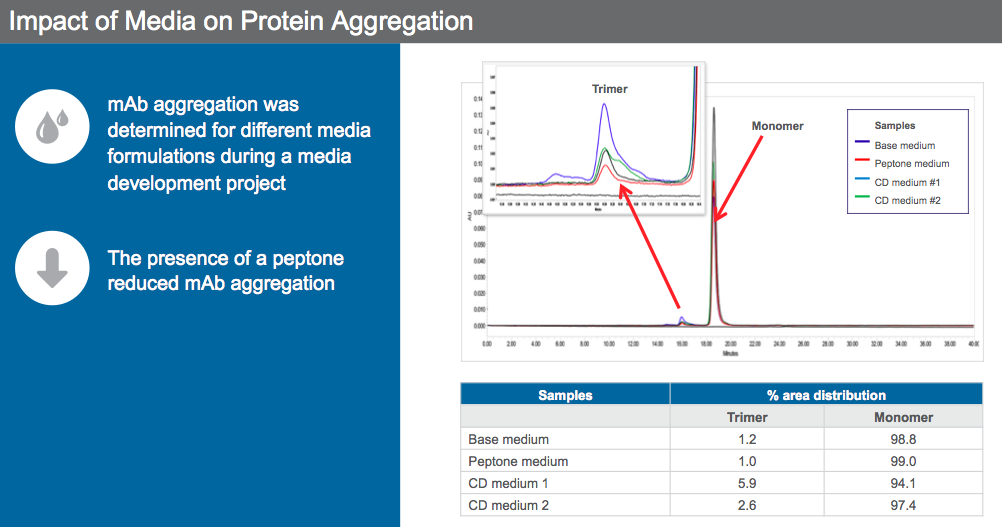
Scientists can leverage media to reduce mAb aggregation. Dr. Sengupta presented data to show how an animal origin free peptone was used for aggregate reduction (Figure 6).
Strategies for leveraging media to achieve desired protein quality attributes
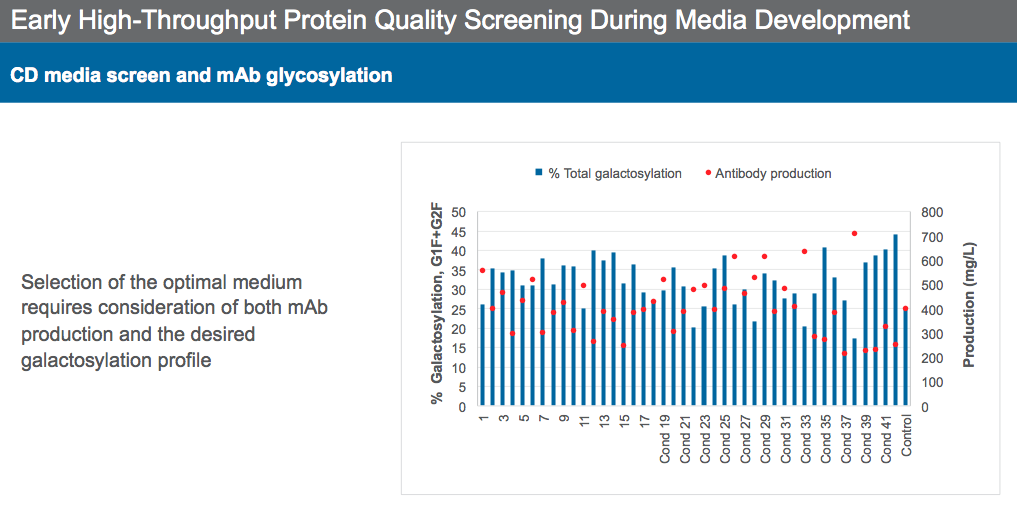
Dr. Sengupta then described strategies to achieve desired monoclonal antibody quality attributes. He began by explaining how high-throughput screening (HTS) can be a powerful tool to select media with protein quality attributes in mind. HTS should be used to evaluate media for productivity, VCD characteristics, and protein quality analytics. It is critical to screen for protein quality while evaluating media so that protein quality issues can be identified early and addressed during media formulation. For example, selecting media solely based on high productivity could create protein quality issues, as a negative correlation was seen between high productivity and lower galactosylation levels in the highlighted case study (Figure 7). Thus, it is important to find the proper balance between productivity and protein quality when selecting a media formulation.
It is also important to understand the media and identify the key drivers for protein quality. Identifying media components that have a strong positive or negative influence on performance and quality can help identify an optimal range to achieve target performance. This can be achieved using mathematical modeling and experimentation.
Dr. Sengupta described the process of identifying key media components and their effect on both productivity and protein quality. Through mathematical modeling and experimentation, media components can be categorized into groups based on their ability to increase/decrease productivity and increase/decrease galactosylation (Figure 8) for the previous example case.
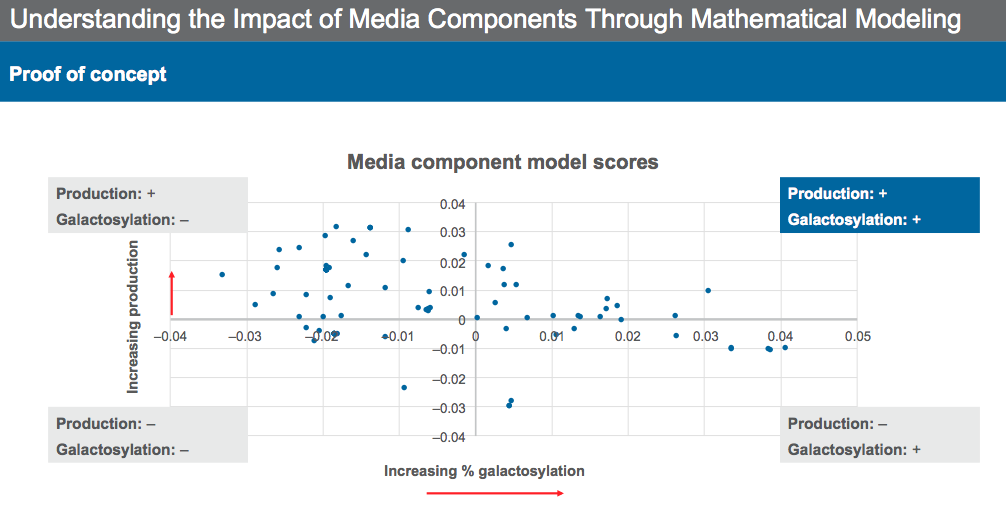
Modeling provides a basis for identifying components that both increase productivity and provide the desired quality profile.
Similar approaches can be used for existing process, for identification of key drivers for production and protein quality. Selected components can then be tested in combination to identify the ideal concentration for formulation (Figure 9) to meet the desired bioproduction goals.
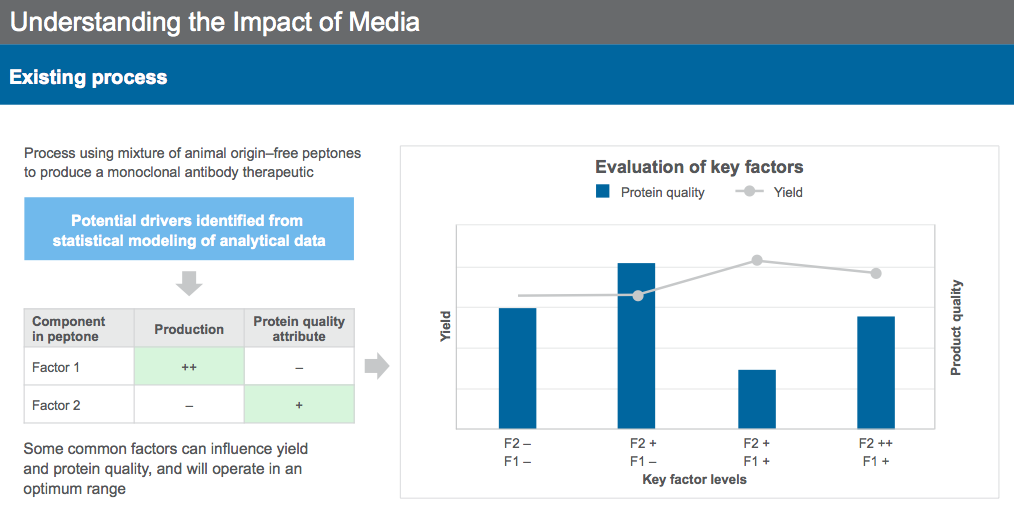
Dr. Sengupta concluded the webinar by reiterating that cell culture media and supplementation provide mechanisms for modulating protein quality in addition to improving yield in the bioproduction process. With an understanding of the drivers of the process, media can be leveraged to achieve the desired protein quality profile.
For more information, please see – www.thermofisher.com/