Optimization of conditions to support efficient HEK293 cell growth and adenovirus production
Viral vector-based vaccines have been a research focus for decades leading up to the recent approval of the vesicular stomatitis virus (VSV) vector-based and human adenovirus-based Ebola vaccines. The SARS-CoV-2 pandemic has further highlighted the continued need for vaccines against emerging and re-emerging pathogens. In response, global vaccine manufacturers have developed several types of effective SARS-CoV-2 vaccines, including adenovirus (AdV) vector-based vaccines. However, the production of large-scale, high-titer viral vector vaccines requires optimization of several cell culture parameters.
As a model system, we infected suspension HEK293 cells with wildtype AdV serotype 5 to assess AdV production using Gibco™ Dynamis™ Medium with Gibco™ EfficientFeed™ C+ (EFC+). We further tested various cell culture and infection parameters and compared cell growth and AdV5 production to an alternative commercially available medium and feed (Medium A and Feed A). As a result, we provide process recommendations for multiplicity of infection (MOI), cell density, and feed concentration to maximize peak adenoviral vector titers.
Materials and Methods
Cell culture conditions
Growth performance was evaluated in simple fed-batch conditions with suspension HEK293 cells in Dynamis Medium (Thermo Fisher Scientific, Cat. No. A2617504). Cells were seeded in triplicate at 0.3×106 cells/mL and cultured in shake flasks (Corning™) at 37°C and 8% CO2. Viable cell density (VCD) was monitored daily for 8 days. Glucose was fed to 6 g/L when levels dropped below 3.5 g/L.
Adenovirus serotype 5 production
Suspension HEK293 cells were adapted to Dynamis Medium or Medium A (alternative commercially available medium) with 10 mg/mL insulin for a minimum of 3 passages. Cultures were incubated in triplicate at 37°C and 8% CO2 and infected with wild-type AdV5 at MOI 0.3 at 1×106 cells/mL and at MOI 10 at 3×106 cells/mL. EFC+ (2X) (Thermo Fisher Scientific, Cat. No. A2503104) was fed at 2.5% or 5%, and Feed A (alternative commercially available feed) at 5% on day 0 (1 hour post infection) and 2 days post infection (dpi). Temperature was shifted to 33°C on 3 dpi. Samples were collected daily, and viral titers were measured with qPCR and TCID50 assays.
Virus quantification using real-time quantitative PCR assay
Cell-associated virus was released after three freeze-thaw cycles of 1 mL cell culture sample [1]. Following centrifugation at 1500 × g for 5 minutes, 200 µL of the top clear supernatant was used for viral DNA extraction using the Thermo Scientific™ GeneJET™ Viral DNA/RNA Purification Kit (Thermo Fisher Scientific, Cat. No. K0821).
Real-time quantitative PCR (qPCR) was performed to quantify the extracted viral DNA in a Roche Lightcycler™ 480 II with Applied Biosystems™ PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Cat. No. A25780) and primers targeting AdV5 Hexon. Viral DNA extracted from human AdV5 (ATCC, VR-1516™) with known particle density was used as reference material to create the qPCR standard curve. The cross-point value of each sample was interpolated against a qPCR standard curve to calculate AdV5 particles/mL.
Virus quantification using TCID50 assay
Total cell lysates after three freeze-thaw cycles were 10-fold serially diluted in Gibco™ DMEM medium (Thermo Fisher Scientific, Cat. No. 11965092) supplemented with 2% Fetal Bovine Serum, qualified, heat inactivated (Thermo Fisher Scientific, Cat. No. 16140071) to create 10-3 to 10-14 dilutions. Diluted cell lysates (200 µL) were inoculated into a 96-well plate seeded with AD-293 cells (Agilent, Cat. No. 240085). After 10 days, cells were fixed with 4% formaldehyde (Sigma-Aldrich™) for 1 hour and then stained with 1% Crystal Violet (Sigma-Aldrich™). After washing with water, the plates were air-dried and calculated for tissue culture infectious dose 50% (TCID50) titer.
Results
Gibco Dynamis Medium supports efficient cell growth and high adenovirus production
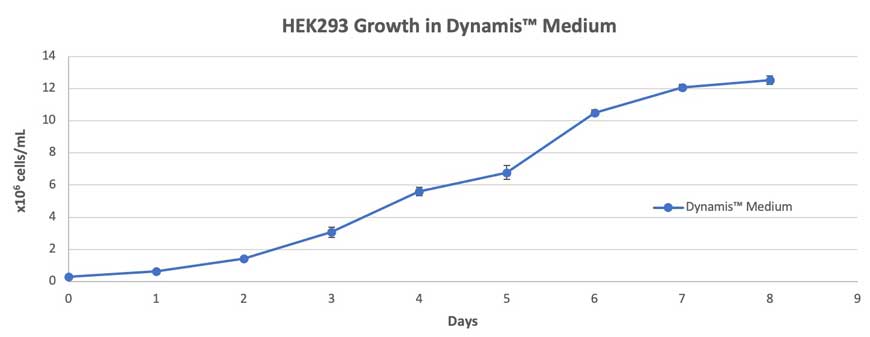
Suspension HEK293 cells cultured in Dynamis Medium showed increasing VCD for more than 7 days. On day 8 post cell seeding at 0.3×106 cells/mL, the VCD was greater than 12×106 cells/mL with log phase growth occurring between day 3 and 6 (Figure 1).
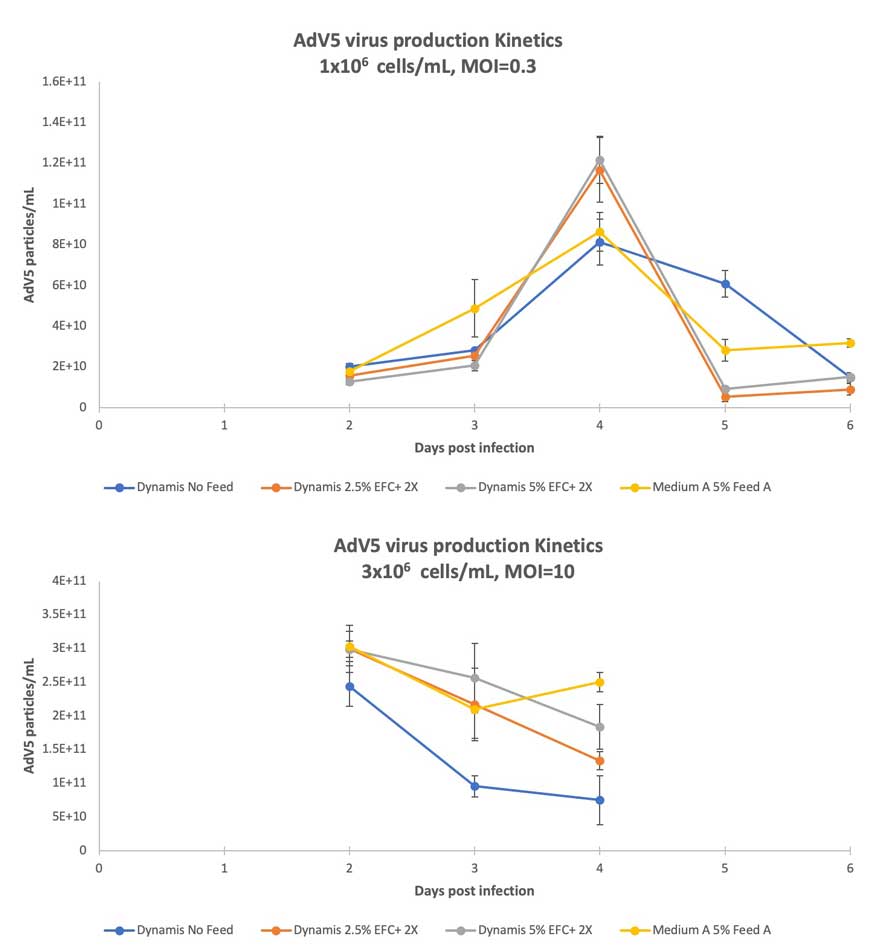
AdV5 production kinetics after low (0.3) and high (10) MOI infections in Dynamis Medium showed high virus titers even without feed addition (Figures 2A and 2B). Addition of EFC+ 2X (2.5% or 5%) further improved titers. The peak titer reached over 1×1011 particles/mL for low MOI and 3×1011 particles/mL for high MOI infections.
Higher peak adenovirus production titer in Dynamis Medium compared to an alternative commercially available medium
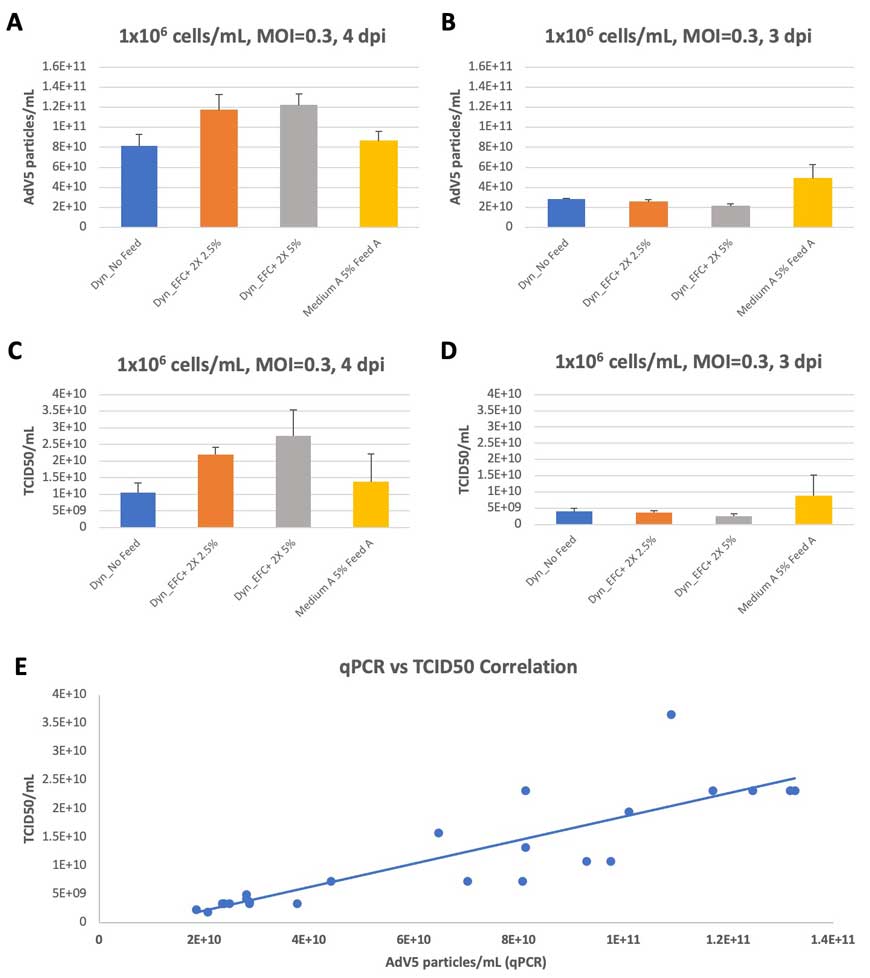
As indicated in Figure 2A, peak viral titer after low MOI infection occurs at 4 dpi for all conditions. At peak titer, the Dynamis Medium with EFC+ 2X (5% and 2.5%) outperformed Medium A with Feed A, as measured by both qPCR and TCID50 assays (Figure 3A and 3C, respectively). The improvement in peak titer was 50-100% higher for Dynamis Medium. On the other hand, viral titers from 3 dpi, prior to the temperature shift and peak viral titers, showed lower titer in Dynamis Medium conditions compared to Medium A (Figure 3B and 3D). These results indicate a more robust effect of temperature shift on virus titers for Dynamis Medium. The qPCR results were confirmed with TCID50 assays, with a strong positive correlation (p<0.001; Figure 3E).
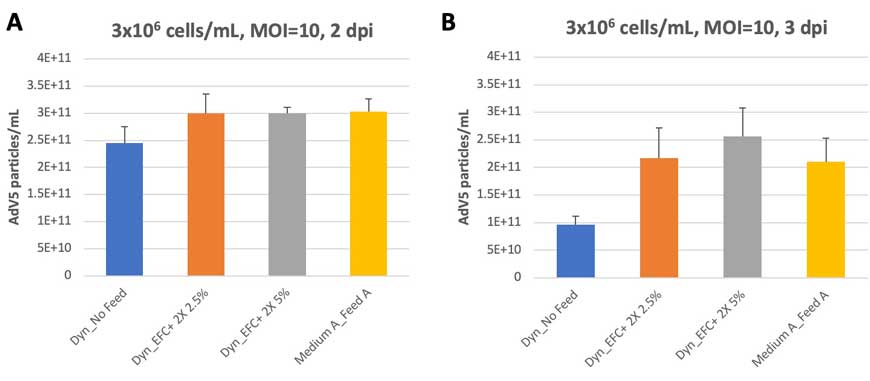
Infection at a higher MOI (MOI 10) resulted in earlier peak titer at 2 dpi with comparable AdV5 titers across both Dynamis Medium (with 5% and 2.5% EFC+ 2X) and Medium A with 5% Feed A. Virus titers on 3 dpi decreased slightly, in line with declining cell viability (data not shown) (Figure 4A and 4B).
Conclusions
In this study, we evaluated HEK293 cell growth and AdV5 production in chemically defined cell culture media. Using Gibco Dynamis Medium and feeding with 5% Gibco EfficientFeed C+ (2X) on 0 dpi and 2 dpi resulted in peak AdV5 titers on 4 dpi for MOI 0.3, and 2 dpi for MOI 10, respectively. Lower MOI conditions benefitted from a temperature shift to 33˚C on 3 dpi. The qPCR results showed that Dynamis Medium with EFC+ achieved high AdV titers, comparable to or better than other tested conditions. TCID50 assays confirmed qPCR results.
Overall, the results demonstrate Dynamis Medium to be effective for suspension HEK293 cell growth and adenovirus production. Optimal conditions may differ depending on the cell line and virus used. As a starting point, we recommend the following process parameters for multiplicity of infection (MOI), cell density, and feed concentration to maximize peak viral vector titers.
Table 1. Recommendations for HEK293 adenovirus-based vaccine production.
| Parameter | Optimized Process for MOI 10 | Optimized Process for MOI 0.3 |
|---|---|---|
| Virus Type | AdV5 | |
| Cell Line | HEK293 | |
| Medium | Dynamis™ Medium | |
| Supplements | 6mM L-Glutamine, 10mg/L insulin (optional) | |
| Feed | EfficientFeed C+ (2X) | |
| Feed Addition | 5% 0dpi | 5% 0dpi and 2dpi |
| Cell Density at TOI | 3×106 cells/mL | 1×106 cells/mL |
| MOI | 10 | 0.3 |
| Time of harvest | 2 dpi | 4 dpi |
| Temperature-shift | N/A | 33˚C 3 dpi |
| Expected titer | >2×1011 vp/mL | >1×1011 vp/mL |
- AdV can be produced with HEK293 cells in Dynamis Medium and EfficientFeed C+ with optional supplementation of insulin, depending on cell line requirements.
- HEK293 cells can be adapted from their current medium to Dynamis Medium through direct or sequential adaptation.
- Cultures should be scaled-up in Dynamis Medium for at least 3 passages before production, to confirm cells are completely adapted to the medium.
- If cells tend to clump, 1% Anti-Clumping Agent can be added to the culture, and cells can be strained during passaging.
- Current growth and infection parameters can be directly transferred to Dynamis Medium and EfficientFeed C+, however, VCD at time of infection, MOI, temperature, and time of harvest.
Footnotes
-
1. Morris SJ, Turner AV, Green N, Warimwe GM. Laboratory-Scale Production of Replication-Deficient Adenovirus Vectored Vaccines. Methods Mol Biol. 2016; 1349:121-35.