
Optimising outgrowth of clones following single cell cloning ensures more predictable and effective cell line development
Successful cell line development is critical to effective CHO cell based biomanufacturing. To accomplish this, companies must identify cell lines that have good manufacturing potential via clone selection. Clones that warrant further investigation are those that are stable, good producers, demonstrating specific attributes based on the product specifications. In order to select the best cell lines, companies must generate a good number of clones for screening.
One challenge of this process is ensuring good outgrowth of clones from single cells in cloning plates. This requires optimisation of the process to ensure the best possible result and that enough clones are generated for screening.
I am pleased to share the following guest blog that presents a guide for optimizing outgrowth from single cells. I was fortunate to be able to interview the author about the article and have provided the transcript of the interview following the guest blog.
Important considerations for optimising outgrowth from single cells in cloning plates during cell line development
A GUEST BLOG BY Dr Ian Taylor, Chief Commercial Officer, Solentim
When developing biotherapeutics, there are many stages of the process that require optimisation. Cell line development is an important upstream part of this process and there are many aspects where changes can have a dramatic effect on timelines and outcomes.
One key area, which is challenging to many of our customers, is outgrowth of clones in their plates following single cell seeding. If conditions haven’t been optimised, a high seeding efficiency will often not translate through into a high number of resultant clones for screening.
Key Factors for Optimisation
CELL LINE CHOICE
This is an important primary consideration, often involving a choice between:
1. Public source of CHO
2. Proprietary commercial cell line e.g. MilliporeSigma, Horizon, Lonza, Celonic
3. Develop in-house from scratch.
Big pharma will generally go for developing a cell line in-house. Smaller and medium-sized companies will usually go for a commercial cell line. The advantage of buying or licensing in a cell line is that it typically comes with defined protocols and recommendations for media and supplements.
The handover of methods for these commercial cell lines can be made even more robust with the use of proven turnkey instrumentation for the key steps, such as the single cell cloning and growth monitoring.
This licensing model has also become less financially onerous with much lower access fees and reduced royalty-based burdens.
CHOICE OF GENE EDITING TECHNOLOGY
Prior to seeding, the cell line must be known to be stable and the chosen methodology must allow for accurate insertion of the GOI into the host genome. For example, we have discussed ATUM’s transposase technology which confers high expression levels and high clone stability, in a previous blog. However, successfully placing a single, edited, high-producing clone in each well of a plate is just the start of a technically challenging process.
SINGLE CELL CLONING METHOD
For instruments that can seed single cells, a major consideration should be the dispensing pressure when single cells are transferred into wells. If too high, the cells will be damaged on arrival which can impact cell survival. Solentim’s VIPS (Verified in-situ Plate Seeding) single cell cloning platform, which uses a much lower pressure than FACS systems when dispensing cells, can result in higher cell survival rates, increasing outgrowth and cloning efficiency.
In Solentim’s recently published case study on Janssen’s use of the VIPS, the instrument was shown to have the same outgrowth rate as seen using gentle manual limiting dilutions, however the number of wells available in which growth was observed increased by 68% [see table].
VIPS has been shown to work well in practice, as illustrated by customers using Horizon’s and MilliporeSigma’s commercial CHO cell lines in conjunction with the VIPS.
GROWTH MEDIA AND SUPPLEMENTS
Depending on the seeding method, single cells can either be dispensed into wells pre-filled with media (e.g. FACS or SCP), or single cells can be dispensed into dry wells and media can then be added on top – as is the case with the VIPS. Commercial growth media is available from several vendors. Further optimisation using supplements can potentially boost cell survival and outgrowth.
Solentim recently announced a partnership with SAL Scientific to address this issue. The collaboration will supply novel media supplements to improve cell growth and Solentim will be the exclusive distributor. Cells grown in chemically defined, animal component-free media can be susceptible to impaired growth and viability, especially when subjected to stress. The SAL ‘Insti’ range of cell culture media supplements are proven to show improved cell growth, colony formation and cloning efficiency. These supplements are suitable for both CHO and HEK cells. See example in Figure 1 for the CHOZN commercial cell line.
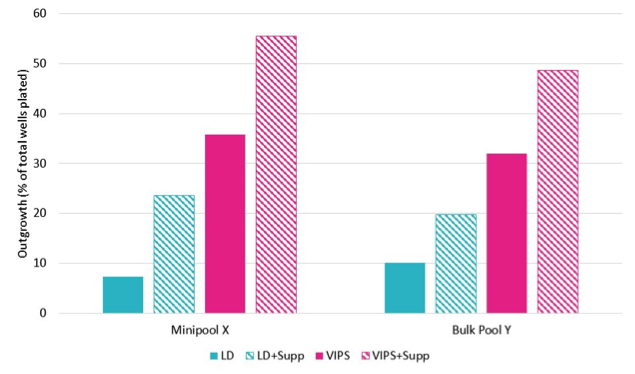
Some customers also dispense cells into a smaller volume initially and then, after a few days, top-up the media as part of a feeding strategy. The top-up can be a different media to the initial cloning media, which supports the cells’ growth once they are through the first cell divisions.
There is a basic requirement that the media and any supplements are animal component-free, and at this stage the media will be different to the final production media. For outgrowth, customers also often employ conditioned media.
However, there is not one method that fits all and conditions need to be tried independently for the specific proteins to be expressed. Even with recommended media, modifications of some key ingredients and the addition of supplements may be able to obtain even higher yields.
WELL PLATE VOLUME
The size of the well and the volume of liquid in the well is also a consideration. We have found that a good number of customers have moved from 96 to 384 well plates for cloning as the smaller well volume seems to encourage cell recovery growth, thus giving rise to better outgrowth.
We have also noted instances where single cells start to divide over the first few days after cloning and then cease to divide further. The reasons for this are unclear but could be related to clone instability.
IMAGERS FOR MONITORING CELL OUTGROWTH
Whole well imagers provide crucial information on which wells to progress further based on outgrowth and single cell origin.
Outgrowth can be assessed in the cloning plates by non-invasive bright field imaging to measure either confluence or ‘total cell numbers’ , and then subsequently after expansion by ‘viable cell numbers’ (for titer measurements) in shaking plates, tubes and scale-down mini bioreactors.
Instruments such as the Cell Metric facilitate imaging during the single cell cloning and clone screening, allowing scientists to monitor outgrowth as whole wells in situ, providing a growth rate curve and percentage confluence for each well, as well as the ability to track back to the single cell clone. It is no good having high seeding efficiency if most of these clones fail to grow into colonies.
Good reliable outgrowth metrics enable scientists to make decisions earlier in the process and to confidently progress potentially fewer clones in fewer plates, saving time and money.
SUMMARY SUGGESTIONS
This blog is not exhaustive and only touches on a number of the key aspects of cell survival and outgrowth optimisation from single cells.
The best advice is to consider using one of the main commercial CHO cell providers as they will provide recommendations and protocols to get you started in-house quickly.
However, to get the best results given changes in expression for different molecules, the use of equipment that gently dispenses cells and gives high survival rates (e.g. the VIPS) along with media optimisation and addition of supplements can literally double the outgrowth and production of these cell lines.
Solentim have recently presented results to show the improvements in outgrowth for HD BIOP3 and CHOZN cell line through modulating changes in background media, as well as adding a novel commercially available animal component-free supplements.
INTERVIEW WITH Dr Ian Taylor, Chief Commercial Officer, Solentim
Why do you feel outgrowth is so challenging?
Fundamentally single cells do not like being on their own in relatively large volumes. Hence they struggle to divide and grow up into colonies. Because of this potential limitation, you have to seed so many more wells and plates to get a sufficient number of colonies.
You have laid out a number of optimization factors. Do you have a suggestion for how companies should approach this optimization? For example is there an order you would suggest, or if they only have time to do a few, which are most critical?
I would suggest vector optimisation for targeted integration and host cell optimisation.
You mentioned the collaboration with SAL Scientific to provide novel cell culture media supplements designed to improve cell growth. Can you tell us a bit more about the products and how this impacts outgrowth optimization?
Yes, these are now on our web site. InstiGRO products are for growth of CHO and HEK cells in the cloning plates.
Can you describe for readers how instruments, like the Cell Metric, can be used to monitor cell outgrowth (they can use cell number in the first instance, then confluence and also colony counting) and also how instruments can be used to make the entire cell line development process more efficient?
The combination of VIPS with Cell Metric means we can isolate a single cell in nanolitre volume and then confirm clonality in the whole well context for the FDA requirements. We can then track the cell division and growth rate in the Clonality Report, which gives an electronic record of the single cell origin and growth of the clone and can be included in the IND.