
PEIpro® Enables Seamless Scale-up of Transient Transfection for Therapeutic Viral Vector Manufacturing
During process development (PD) for virus vector production, choosing raw materials from reliable suppliers that can provide the highest quality to meet Advanced Therapy Medicinal Products (ATMP) GMP guidelines is key and will facilitate transition to large-scale clinical-grade virus manufacturing. With the launch of commercially available PEIpro®-GMP, Polyplus-transfection® SA now provides a complete PEIpro® product range to address concerns often raised with transient transfection for large scale manufacturing of viral vectors: efficiency, reproducibility and scalability.
Seamless scale-up of transient transfection for large scale manufacturing
PEIpro® product range is composed of three quality grade reagents (PEIpro®, PEIpro®-HQ and PEIpro®-GMP) for each step of nucleic acid-mediated viral vector-based manufacturing. This allows you to strictly adhere to Quality Requirements for manufacturing of ATMPs with the availability of an identical transfection reagent at three quality grades: PEIpro® for initial Process Development, higher quality grade PEIpro®-HQ for pre-clinical and early stage clinical stages, and PEIpro®-GMP for late clinical stage and commercialization. Large-scale transfection protocols established with PEIpro® during process development are guaranteed to be seamlessly applicable during manufacturing of viral vector clinical batches using PEIpro®-HQ and PEIpro®-GMP (Figure 1).
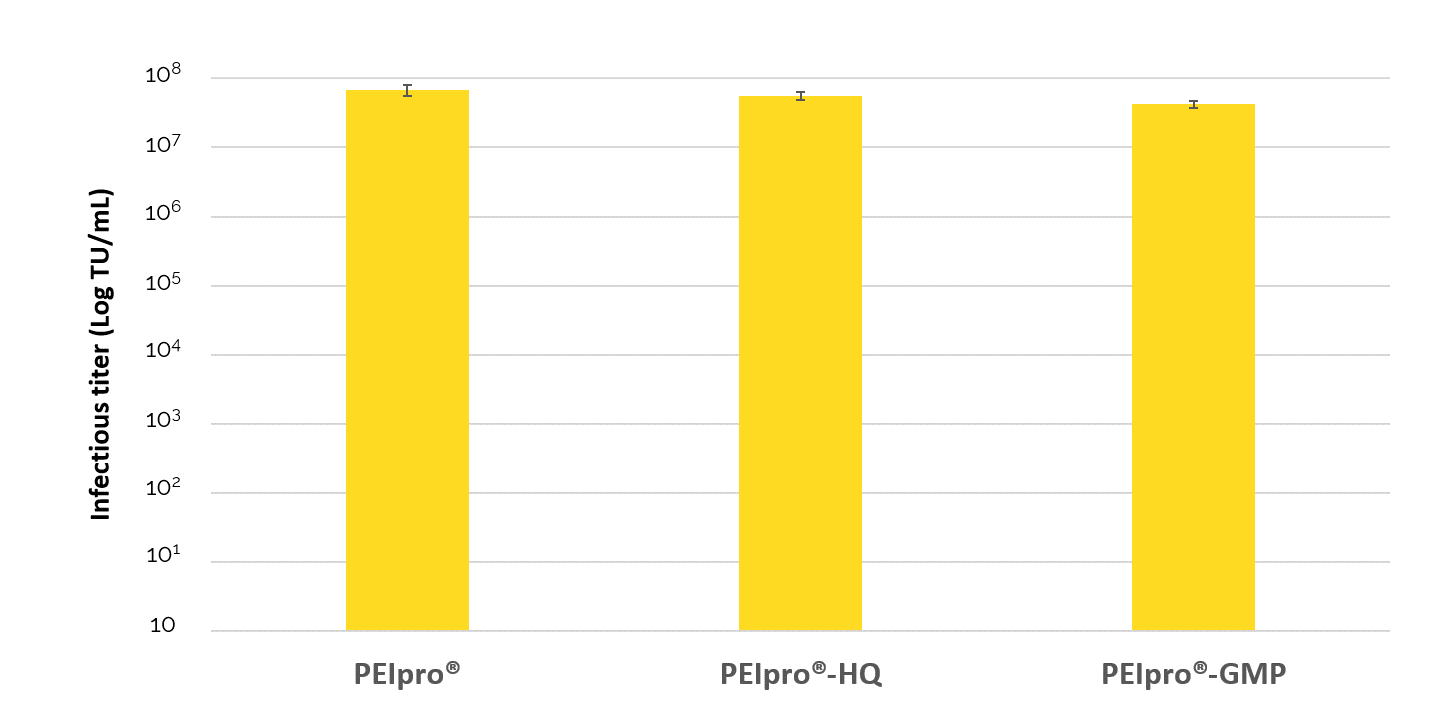
PEIpro® product range addresses transfection challenges: efficiency, reproducibility and scalability.
With its unique property to efficiently condense several plasmids of different sizes, PEIpro® is ideal for optimal co-transfection of several plasmids containing the gene of interest and necessary viral components to produce full recombinant virions. PEIpro® has benefited from extensive developments and is therefore the unique PEI-based transfection reagent that can offer the flexibility and robustness that is needed during PD and scale-up; it is compatible with commercially available adherent and suspension virus production systems, particularly for AAVs and lentiviruses (Table 1).
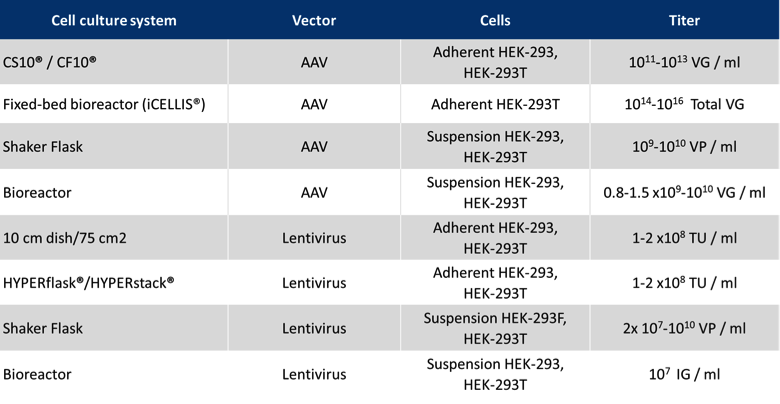
PEIpro®-GMP fulfills current GMP guidelines for ATMPs
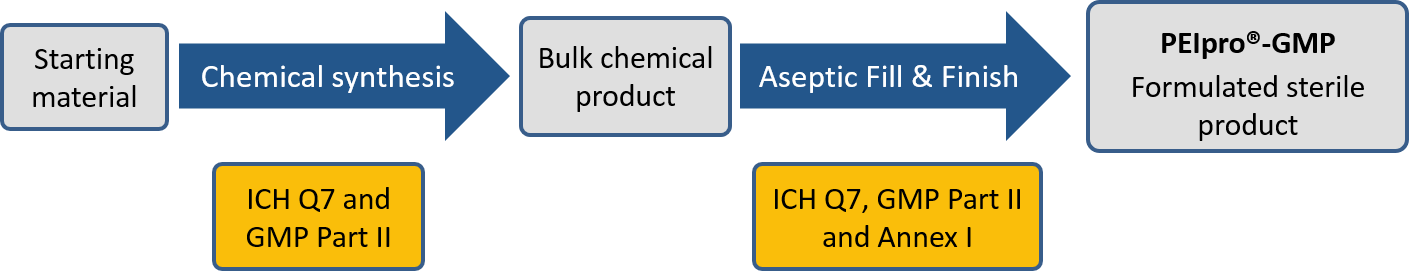
PEIpro®-GMP is the highest quality grade and the only commercial PEI transfection reagent supplied in a bag with MPC connectors and weldable tubing. It is strictly manufactured according to a validated manufacturing process in compliance with GMP guidelines to ensure traceability from starting material to the final product (ICH Q7 and Eudralex Vol 4, Part II, Annex I). Both steps of PEIpro®-GMP manufacturing (chemical product and fill & finish) are managed in compliance with GMP guidelines in GMP accredited facilities (Figure 2).
To learn more, please visit https://www.polyplus-transfection.com/products/peipro/