
Purposeful design of a next-generation single-use film for optimized performance in biomanufacturing
In this mini-webinar, Susan Burke, PhD, Bioprocess R&D, GE Healthcare, Life Sciences, presents the process in which GE Healthcare developed a next-generation single-use platform film, Fortem™. Fortem is available across GE’s entire portfolio of single-use products and was designed to maintain critical performance attributes, such as container integrity and gas barrier properties, under the significant forces exerted during bioprocess operations.
Film Design
Dr. Burke began her talk by discussing the complex demands of bioprocess films. One of the challenges with bioprocess film to date is that it has been mostly “borrowed” from other applications and thus wasn’t developed specifically to support bioprocess operations. By developing a new film, GE Healthcare set out to close some film performance gaps and to truly create a film that could serve all the required functions for single-use film throughout biomanufacturing.
Susan then presented some of the areas that GE wanted to address in the design of a platform film:
- Simplicity: reduce verification and qualification requirements with single film platform
- Material science: meet critical attributes for sensitive applications
- Performance: optimize for a wide range of applications
- Security of supply: mitigate risks through supply and quality control, from resin to assembly manufacturing
In addressing simplicity, Susan explained that as single-use technologies have become more mainstream in biomanufacturing, it has also become more time consuming and burdensome to conduct product qualifications for each single-use product used in a process. Utilizing a platform film enables a single qualification for multiple single-use products in a process.
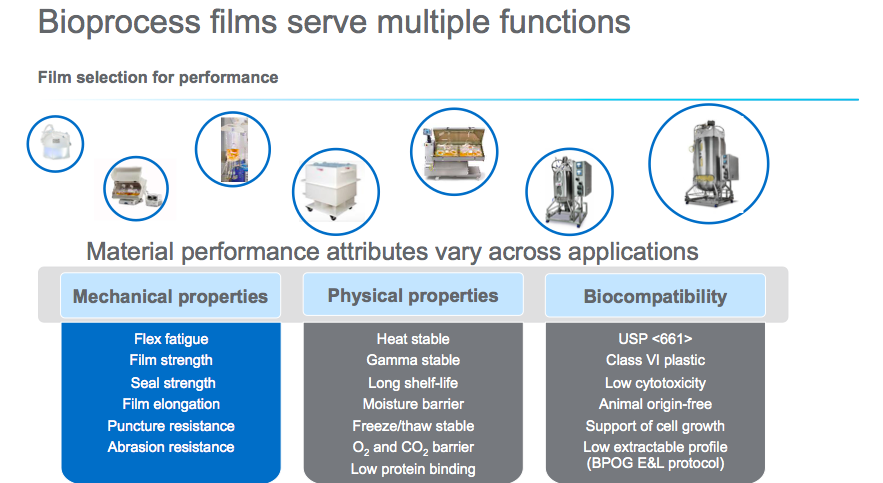
Susan continued by describing how in order to create a film that could truly be used in a platform approach, the film would need to serve multiple functions. In Figure 1, Susan highlights the key performance attributes that were included in the design planning. One challenge that the team faced was in balancing the performance attributes. In some cases the performance attributes would run counter to each other and adjusting one would have a negative impact on another.
Film Manufacturing Partner
To accomplish the task of creating a film to meet all these needs, Susan discusses the need for a strong partner with a successful film manufacturing history. GE Healthcare partnered with Sealed Air Corporation, a manufacturer of primary films for pharmaceuticals, in the design and manufacturing. In addition, GE material science experts worked closely with customers to design a film that would meet the critical quality attributes required for a platform bioprocess film.
Commitment to Legacy Films
Susan then explained that while the goal was to create a new single-use platform film for all products in their single-use portfolio, GE was also aware that they currently had a single-use portfolio that incorporated several different films. This was largely due to the acquisition of new products that utilized existing films. GE has made a commitment to continue to support their legacy films because they realize this is critical for customers already using these films, but they are excited to offer a platform strategy for new product initiatives.
Film Development
Next Susan discusses the development of the new film and the primary principals used. The most important of these was to fully understand the needs of the biomanufacturing process and their customers. GE Healthcare worked closely with customers and industry associations to gain feedback on critical quality attributes and performance demands.
GE focused on these principles in the development approach:
- Define performance needs and specifications
- Establish resin selection criteria (e.g., animal origin free, EP compliance)
- Leverage knowledge of bioprocess needs
- optimized antioxidant package
- Thorough analysis of raw materials
- physical and mechanical properties
- extraction profile
Film Construction Materials
In material selection it was important to take into account film performance, versatility and durability. It was critical that each layer be comprised of material that met the specific needs of that layer. In the talk, Dr. Burke describes the unique properties of each layer. Here are some main points:
- The fluid contact layer, polyethylene and cyclic olefin copolymer (COC) is compliant with EP 3.1.3, EP 3.1.5, JP 7.02, USP Class VI and also COC acts as a macromolecular slip agent, eliminating the need for traditional small molecule additives.
- The gas barrier has two different types of EVOH (alcohol substitution) incorporated into the structure that provides a barrier to gases in both wet and dry conditions.
- The outer layer is comprised of a specific nylon chosen to provide strength even in humid conditions. The interior layers are composed of a polyethylene blend for robustness and flexibility over a wide temperature range.
- There is also an antioxidant package, which is a selection of additives and placement in film optimized for minimal impact on mammalian cell culture performance.
Film Architecture
In addition to material selection, film architecture was also very important. Again the issue of balancing attributes was important, as it was critical that the film provide both robustness and flexibility. Susan shared two examples where unique forces put pressure on the bags to remain strong yet flexible – rocking bioreactors and liquid transportation. In order to achieve this balance, they knew that they would need to place the most rigid layers in an area where they would get the least amount of flex. They calculated the neutral plane between the outside tension and inside compression. By putting rigid layers like the gas barrier layer in the neutral plane, they were able to protect these layers from cracking. This provided the robustness required, while also allowing the film to be flexible in the outer layers.
Film Optimization
To ensure the new film met the most physically demanding applications, it was optimized for two of the most stressful on film – liquid storage and large-scale manufacturing. Susan explained that they identified and ensured that the film met key requirements for both liquid storage performance and large scale biomanufacturing operations in large vessels.
Key requirements met for liquid storage performance:
- Low extraction for minimal impact on fluid properties
- Remain integral through freeze/thaw down to -80°C
- No deformation or integrity loss when exposed to static and dynamic stresses
Key requirements met for large-scale biomanufacturing in Xcellerex vessels:
- Strong performance through temperature range for microbial fermentation (up to 60°C)
- Durability with large mixer and bioreactor applications
Film and Cell Culture Performance
To confirm that the film performed well when testing in cell culture applications, she explained that they utilized a cell culture screening methodology that included a mAb-producing CHO DG44 cell line, which is sensitive to bDtBPP down to 0.1 mg/L (100 ppb). Results demonstrated that population doubling time and cell viability were quite strong with Fortem. This validated the results of the extraction profile, which showed bDtBPP below levels of detection.
Security and Supply
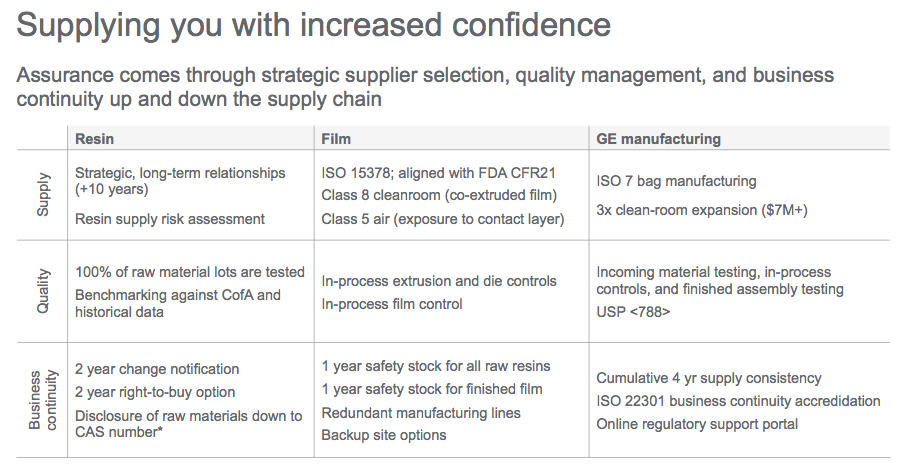
Susan stated that GE believes assurance comes through strategic supplier selection, quality management, and business continuity up and down the supply chain.
To safeguard consistent performance and protect from supply disruptions, GE Healthcare developed a business continuity plan which is ISO22301 accredited. It includes robust site preventative and recovery plans, a ten-year strategic supply agreement, and strategic safety stocks in both raw materials and finished film. More on security and supply measures can be seen in table 1 from the webinar.
Investing in the Future
Susan closed the webinar by talking about the steps GE Healthcare has taken to be certain they are meeting customer needs and expectations now and in the future. One of these steps includes active participation with industry associations like BPOG and BPSA. Another step is in ongoing investment globally and across the business, with recent investment of over 40 million USD in capacity, quality controls and security.
Summary
In summary, Susan shared that by using purposeful design coupled with material science and application knowledge, GE was able to create a cornerstone film to meet industry needs now and in the future.
For full data and more information, please watch Dr. Burke’s presentation below:
About the Presenter:
 Susan Burke, Ph.D., Staff Scientist, Materials Science, GE Healthcare
Susan Burke, Ph.D., Staff Scientist, Materials Science, GE Healthcare
Susan holds a Ph.D. in Materials Chemistry from McGill University and has expertise in polymer technologies for healthcare applications. Prior to joining GE Healthcare, she spent more than ten years in various R&D roles within the pharmaceutical and medical device industries overseeing product and process development initiatives.