We are in process development on using a closed system to produce adherent cells. What steps would you take to be sure you are starting with a contamination free culture before putting it into the closed system. Also would you test during culture or wait until harvest. At what points in production process do you advise people to test for contamination?
This question is part of the following Ask The Expert session:
How to minimize contamination risk and protect your cultures
Answered by:
Company: Corning
Job Title: Scientific Supporting Specialist supporting EMEA regions
Answer
Bioburden control is required/crucial for key applications, as example during pharmaceutical drug manufacturing processes.
Aside from testing raw materials and media sterility at the very beginning of the process, some “in-process testing” can be suggested as below:
- Sterility test every time the culture is passaged.
- Sterility test of inoculum before inoculating any vessels (or large scale bioreactor that is used as final production step).
- Have a back-up cultures for the seed train process (seed train process = generation of an adequate number of cells for the inoculation of a production bioreactor. Ref: Seed train optimization for suspension cell culture. T. Hernández Rodríguez et al. BMC Proc. 2013; 7(Suppl 6): P9.)
Typically, a small sample of the culture can be taken and inoculated within trypticase soy broth tube (or agar).
These tests are performed during 14 days at 37 and 25 degrees Celsius. As time frame of 14 days can be not feasible during production processes, test sterility of the inoculum for a minimum of 24 hours can be evaluated on site.
In addition, besides incubator standard procedures to ensure incubator sterility (please refer to question 1 within this page), suggestions can be shared for open operations in the biological safety cabinets (BSC) as below:
- Agar plate testing of operator gloves directly after sterile gloves are put on and before beginning the process
- Environmental testing in the BSC – open agar plates are placed in the BSC during cell culture processing
Below is an image of a holistic approach to microbial control strategies. For a more extensive overview, we like to invite you to revise “L. Anastasia. Microbial control strategies in bioprocessing falling short of assuring product quality and satisfying regulatory expectations. American Pharmaceutical Review 16(2) · March 2013”
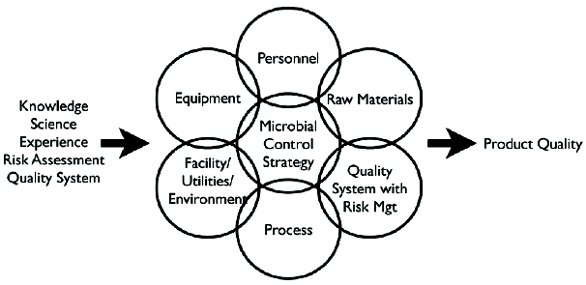
We have many Field Applications Scientists at Corning who have extensive experience with specific bioprocess and closed system approaches. If you have more application specific questions please contact one of the following regional Scientific Support groups to be put in contact with your local Field Applications Specialist. Americas: Scientificsupport@corning.com, Greater Asia (including Greater China, Southeast Asia, Korea, India, Australia and New Zealand): ScientificSupportAP@corning.com, EMEA (including Europe, Middle East and Africa): ScientificSupportEMEA@corning.com, Japan: ScientificSupportJP@corning.com