
Scalable High Yield System for Mass Culture of Attachment-Dependent Cells
As increasing numbers of cell and gene therapies progress from research to clinical trials and on to commercialization, the need for efficient manufacture of high numbers of cells is critical. Many of the cells used in these therapies are attachment-dependent, which means that they need a surface on which to grow and expand. This need for attachment makes growing them in large numbers more challenging, as adherent systems do not traditionally scale up to the high volumes that can be achieved with suspension systems. However, suspension systems typically require more time and resources to develop a successful process.
In addition to scalability, successful production systems will be able to achieve high cell yield in a small footprint. Efficient scale-up should not require a substantial increase in footprint, which would require larger manufacturing facilities and more capital investment.
Lastly, attachment-dependent cell production ideally would take place in a closed system. A closed system utilizes equipment designed for and operated to ensure that the product is not exposed to the room environment. Materials may be introduced to a closed system, but the addition must be done in such a way to avoid exposure of the product to the room environment. One of the biggest and most easily attained benefits of implementing a closed system, whether in research, pilot or large scale, is in reducing the risk of contamination by viruses or other adventitious agents.
Case Study – Maximizing Yield in Vero and HEK293T Cell Lines
Corning recently published an excellent Application Note, “Maximizing Yield for Attachment-dependent Cells with the Corning® CellCube® System”. In the application note, they present a case study that demonstrates how the CellCube System efficiently scaled up manufacture of two commonly used attachment-dependent cell lines, Vero and HEK293T.
We were fortunate to be able to interview the authors of the application note and have included the interview transcript below.
The CellCube System
The Corning CellCube system provides a simple, compact, and scalable method for mass culture of attachment-dependent cells. Each CellCube module consists of a series of parallel, polystyrene plates joined to create thin, sealed laminar flow spaces between adjacent plates. CellCube modules are available in three basic sizes (Table 1). The 10- and 25-layer modules are comprised of 10 and 25 culture plates, respectively, while the 100-layer module is made up of four 25-layer modules series. As gas-conditioned culture medium is circulated through the CellCube, design of the modules allows for distribution of nutrients and oxygen with low differential gradients across all cells within the modules.
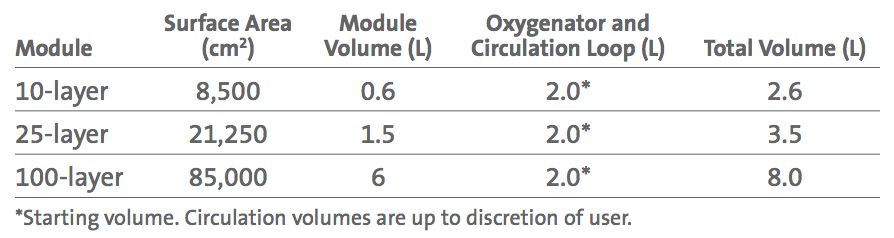
Study Design
In the current study, the CellCube system was used for expansion of two commonly used bioproduction cell lines, HEK293T and Vero. The basic closed system was configured with a peristaltic pump to drive continuous circulation through the CellCube 100-layer module and paired with the Eppendorf BioFlo® 120 (controller; Eppendorf B120ACS000) with a BioBLU® 3c single-use bioreactor (SUB; Eppendorf 1386000300) for medium conditioning. The controller simplified oxygenation and pH control of the circulating medium. Medium conditioning in the SUB was sufficient to sustain both HEK293T and Vero cell growth in the CellCube 100-layer module for 5- and 6-day expansions, respectively. Final harvest yields for both HEK293T and Vero cell lines greater than 10 billion cells demonstrate the utility of the CellCube system for mass culture of adherent cell lines with efficient medium usage.
Highlights from the Case Study
Closed System for Culture and Media Conditioning
The culture medium (approximately 2L) within the system is removed from the SUB by a peristaltic pump and is then pumped into and distributed throughout the Corning CellCube 100-layer module. Medium flows from the outlet of the CellCube module, back to the SUB for conditioning. The controller automatically controls pH via NaHCO3 and CO2. Dissolved oxygen (DO) in the medium is also maintained by the controller, which continuously refreshes the gas mixture supplied to the headspace of the SUB and sparged directly into the medium. Fluid flow and gas exchange within the SUB are carefully controlled to help eliminate turbulence, foaming, and to prevent protein degradation. Addition of antifoam in the culture medium aids in reducing medium foaming (Figure 1). *Peristaltic pump, controller, and SUB are sold separately.
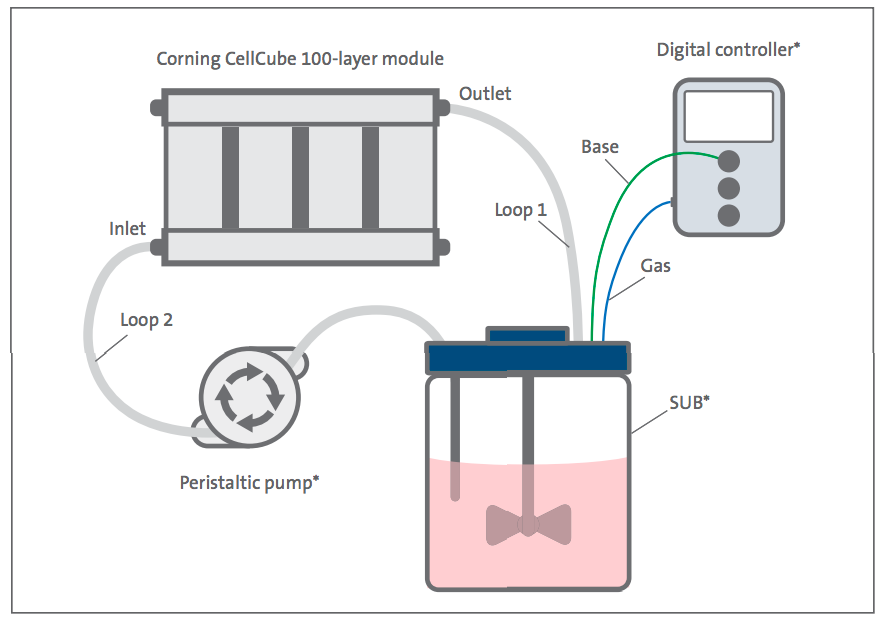
Efficient Cell Seeding
The CellCube 100-layer module was used as the culture platform for expansion of HEK293T and Vero cell lines with medium conditioning in a SUB (Figure 2). Cells were seeded onto the CellCube module via a single seeding. For the single seeding, pre-equilibrated medium was removed from the CellCube module, inoculated with cells to seed the entire 8.5 x 104 cm2 surface area and returned to the CellCube module. The vessel was rotated into a vertical position to seed the first side of the polystyrene culture plates (Figure 3). Historical data indicated 20- to 30-minute attachment time for HEK293T and Vero cell lines on TC-treated vessels so the initial seeding was chosen as 20 minutes for the first side of the vessel. Each subsequent rotation was incubated for 20 to 30 minutes for a total of two incubations per side. Some cell lines, such as mesenchymal stem cells (MSCs), may need an extended seeding period.
High Cell Yield Requiring Minimal Operator Intervention
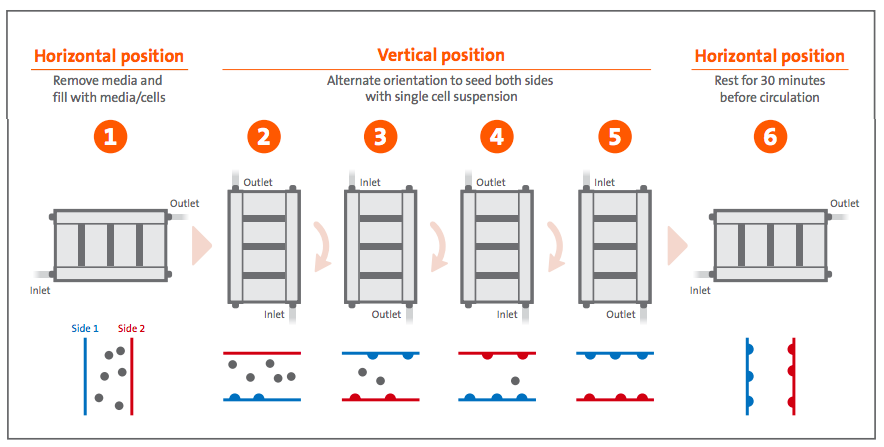
Both HEK293T and Vero cultures expanded in the Corning CellCube system with minimal intervention. The controller provided tight control of temperature, pH, and dissolved oxygen, which adequately conditioned the circulating medium in the SUB. Daily offline medium analysis was used to monitor pH and dissolved gasses. As necessary, the system pH was recalibrated according to the offline values. In addition, glucose depletion and lactate accumulation were tracked via medium analysis to determine the day of harvest (data provided in application note). For the HEK293T cultures, medium glucose levels began to decrease between Day 3 and Day 4 of culture as lactate levels began to rise (data provided in application note). Vero expansion progressed more slowly, with steady glucose depletion coincident with lactate accumulation from Day 3 through 5 (data provided in application note).
Metabolite concentration proved to be a predictable determinant of cell confluence, as final harvest yields were comparable between different expansion runs for the same cell type. Total HEK293T harvest averaged 1.4 x 1010 ± 1.4 x 109 cells with >97% viability (Figure 6A), which corresponds to 1.6 x 105 ± 1.9 x 104 cells/cm2 or 33 ± 4X expansion (Figure 6B). Total Vero harvest averaged 1.1 x 1010 ± 3.6 x 109 cells with >97% viability (Figure 6A), which corresponds to 1.3 x 105 ± 4.3 x 104 cells/cm2 or 26 ± 9X expansion (Figure 6B).
High Yield from Compact Footprint
This yield from such a compact footprint is notable, given the number of standard planar culture vessels necessary for production level scale-out to the same surface area (Table 3). For example, the total surface area of four Corning CellCube 100-layer modules is equivalent to several dozen multilayer stacked culture vessels, or 400 roller bottles. Using many stacked vessels would require significant incubator space or warm rooms and racks for roller bottle culture. Not to mention, the ergonomic challenge of handling so many culture vessels versus the compact CellCube system. The entire closed system with peristaltic pump, including the controller and SUB required minimal space, such as a 3-foot x 5-foot area in the warm room. Certainly, the total area would vary depending upon the size of the digital controller with SUB chosen for medium conditioning and the peristaltic pump.
Scalability
Corning studies demonstrate that optimization of media formulations and culture parameters in the smaller CellCube 10-layer module can easily be scaled to the CellCube 100-layer module. Thus, the linear scalability of the CellCube system enables transition from research through process development to manufacturing.
Interview with study author, Ann E. Rossi, PhD
What other cell lines have been successfully produced in the CellCube?
In our laboratory, we have successfully cultured Vero, HEK293T, and MDBK cells. There are also several peer-reviewed publications – cited in the Application Note – that evaluate expansion of BHK, CHO, MRC-5, COS M6, SKNMC, TE Fly GA18, and Phoenix Frape-1 and Phoenix Frape-3.
Could you describe the ideal scale for production using the CellCube?
The ideal scale and configuration using the CellCube is cell line- and process-dependent. The optimal scale for each process can be tailored by manifolding modules in different configurations, changing the size and/or number of bioreactors for medium conditioning, and adjusting perfusion rate to meet cell requirements for gas exchange, nutrient delivery, and metabolite waste removal.
How does cell health compare in the Cell Cube to other types of culture systems?
Medium circulation or perfusion in the CellCube system continuously feeds cultures with conditioned medium, which is ultimately better for cell health. Based on historical data, cell health is as good if not better in the CellCube system versus other adherent cell culture vessels. However, we have not directly compared the health of cells expanded in the CellCube system versus other large-scale adherent platforms.
From a workflow perspective, how easy is CellCube operation and training?
The Corning CellCube system is simple to both learn and use for mass culture of adherent cells. CellCube system operation can be learned quickly with basic knowledge of general adherent cell culture and closed bioprocess systems. Most of the time spent on training involves learning the setup and operation of the bioreactor controller system (sold separately). Once trained, one single operator can run a CellCube 100-layer process from equilibration through cell harvest.
What was the most exciting aspect of the study in terms of production?
The most exciting aspect of this study was the predictability by which we could determine harvest date based upon metabolite measurements. In preliminary studies, we would seed at a specific density and harvest X-number of days later based upon confluence of our companion T flask cultures. We were not getting the expected yields. Once we started to rely on glucose and lactate measurements, we got reliably high yields. Though this strategy might not work for all cell types, we simply relied on the system to tell us when it was ready for harvest!
ABOUT THE INTERVIEWEE:

Ann E. Rossi, PhD
Dr. Ann E. Rossi graduated from the University of Rochester School of Medicine and Dentistry with a Ph.D. in Pharmacology and received postdoctoral training at the University of Chicago. Prior to joining Corning, Ann worked as a Senior Scientist at ARMGO Pharma, Inc., a small private pharmaceutical company, contributing her expertise in calcium signaling toward developing new assays for the company’s screening cascade. Ann began her career with Corning Life Sciences as the Applications Lab Manager where she utilized her strong academic and industry research experience to direct the activities of the Applications Laboratory. Currently, Dr. Rossi is the Senior Bioprocess Applications Scientist, functioning as the technical lead for generating applications in support of Corning Life Sciences’ Bioprocess and Cell/Gene Therapy portfolios including CellCube and HYPERStack platforms.
To learn more, please attend the webinar: Maximizing Yield for Attachment-dependent Cells with the Corning CellCube System, presented by Dr. Rossi
For more information, please see Corning CellCube Culture System
To read application note, Maximizing Yield for Attachment-dependent Cells with the Corning® CellCube® System