Spheroid Cell Culture – Practical solutions for frequently asked questions
The past decade has seen an increase in publications recognizing the value of culturing cells in three dimensions (3D) and the use of these 3D cell culture models has shown utility in many areas of research from cancer biology to regenerative medicine. One of the most common 3D cell culture types is spheroids. Spheroids more accurately recapitulate the native in vivo microenvironment with respect to cellular function and response to drugs than more traditional two-dimensional (2D) models. That said, working with spheroids is not without its challenges. Many labs working with spheroids have run into technical hurdles incorporating them into existing cell culture workflows. Whether it is issues with handling or optimizing protocols to assaying cells in 3D, these obstacles can be overcome.
In an effort to investigate some of the most frequent challenges and provide useful tips for spheroid culture, we talked to Cindy Neeley, Leticia Montoya, and Bhaskar Mandavilli from Thermo Fisher Scientific about what they see as the six most frequently asked questions about culturing spheroids and to provide our readers with practical solutions to these issues.
Our questions and answers are below.
Q1: Controlling the size of 3D cell models can be a real challenge. How do I consistently grow uniform spheroids to get repeatable results?
The easiest way to control the size of spheroids is by adjusting the initial cell seeding densities. However, this approach can only work when a single spheroid is reliably formed from a given number of cells. A recommended approach for such application is to avoid culture vessels with large surface area such as a T-flask or a large petri dish. Instead, a more confined physical space to promote spheroid formation, such as a round bottom microplate (e.g. 96-well U bottom plate) would be more suitable. There are several ways to consistently generate uniform spheroids in a microplate format as described in Figure 1, each with their advantages and disadvantages. The system selected will vary based on cell type and research needs.
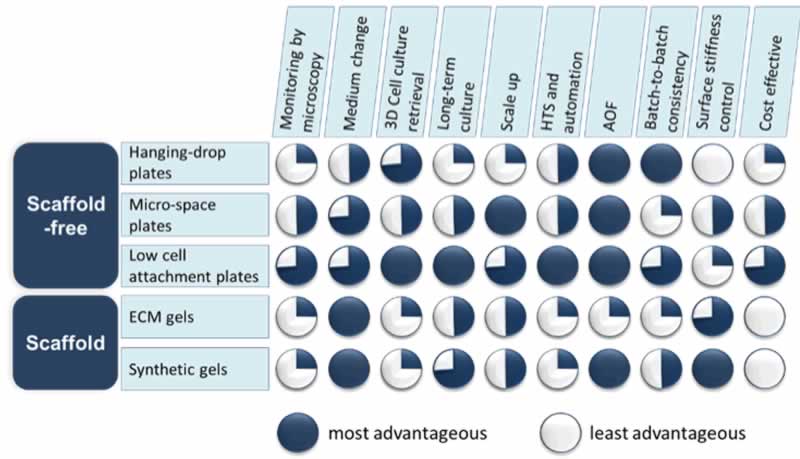
The hanging-drop method is one of the first used to establish cellular aggregates. This is a time-consuming technique requiring skilled hands to execute which may not be practical for many laboratories. There are commercially available hanging-drop plates with user-friendly designs to allow for a greater rate of success; however, this method is not well-suited for long-term spheroid culture as frequent media change in the suspended droplet is challenging. Additionally, high cost and low output of these plates has hindered their adoption by most research labs.
More recently, micro-patterned plates have become popular in the field of 3D cell culture particularly for high throughput screening (HTS) protocols. A micro-patterned plate is a plate that contains many micron-sized wells within one well allowing for multiple spheroids to be grown in one shared environment, which can maximize the data output when used in the drug discovery process. While large numbers of spheroids can be generated with relative ease, both the uniformity and morphology can vary greatly from well to well, making it difficult to obtain a homogenous culture. Furthermore, long-term spheroid growth and differentiation in the micron-sized wells present challenges to researchers in the field of regenerative medicine and disease modeling.
Alternatively, some scaffold-based protocols recommend coating standard cell culture plates with an extracellular matrix (ECM) or synthetic gel prior to seeding cells. The scaffold materials limit the movement of cells in the wells, promoting aggregation into a single spheroid. Monitoring cell growth over time as well as retrieving spheroids from the scaffold for further analysis may require extra steps that are usually time consuming and labor intensive. In addition, the potential biological impact of the ECM components, some of animal origin with lot-to-lot inconsistency, must be carefully considered as well as the fact that the coating and dissolving of the ECM/gel can be problematic in an automated setting.
A much simpler and more effective way to achieve uniform and single spheroid formation is the use of the low cell attachment plates where no scaffold is needed. The low cell attachment surface inhibits cell attachment to the culture ware by blocking the attachment of ECM proteins in the culture system (i.e. proteins in sera, endogenously secreted ECM, etc.). This combined with the geometric shape of the wells promotes the cells to aggregate into a single spheroid in each well. It is an easy-to-adapt and affordable system compatible with HTS platforms. The caveat is that the plastic surface modifications of such low cell attachment plates have to be superb since imperfections result in cell attachment to the bare plastics. Therefore, choosing a reputable manufacturer with surface modification expertise and good quality standards is highly recommended. Once a reliable low cell attachment surface is selected, it is not difficult to incorporate the device into routine cell culture for the formation of uniform spheroids, enabling researchers to control the size of their 3D cell models to generate comparable and meaningful results time after time.
Q2: How do low cell attachment plates support spheroid culture? And how do they compare to the non-treated polystyrene plates for the suspension 3D culture?
Compared to non-treated polystyrene plates, low cell attachment plates, such as Nunclon™ Sphera™ 3D cultureware (Thermo Fisher Scientific), are able to better support the formation and growth of spheroids consistently and reproducibly. The low cell binding surface offers a superior solution to efficient spheroid formation with fewer satellite colonies, thereby improving the homogeneity of the spheroid culture, key to achieving reproducible experimental results (Figure 2).
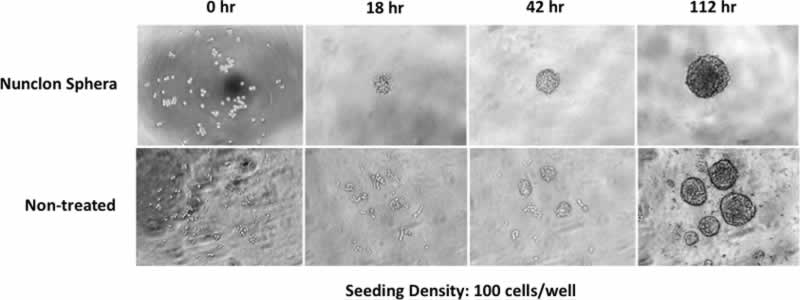
The Nunclon™ Sphera™ 3D culture plates are user-friendly and easy to incorporate into current cell culture workflows. However, there are some tips and tricks that can greatly improve success in obtaining a homogenous spheroid culture, which will be discussed in subsequent questions.
Q3: What can I do differently when my cell lines do not form compact spheroids in the wells of the microplates?
It should be noted that not all cell types will readily form tight spheroids. However, there are some helpful tips and tricks that can support spheroid formation when using the Nunclon™ Sphera™ 3D culture plates. First, after seeding the cells, centrifuge the plate at a low speed (e.g. 150 x g for 5 min). This will help the cells quickly settle at the bottom of the wells. If the cells are fragile, use caution when selecting the optimal speed and condition for this centrifugation step.
Also, cell types can vary the rate in which they form spheroids. Some cell types will readily form spheroids within hours, while others require several days to form compact spheroids. For these situations, consider replacing ½ volume of the media with fresh media every 2-3 days to maintain culture health during spheroid formation.
Q4: Do you have any tips for best practices to handle spheroids?
Most importantly, care needs to be taken when working manually with the spheroid cultures. Half media changes may be required for long-term culture or differentiation protocols. To do so, carefully tilt the microplate at an angle so that the pipette tip does not touch the bottom of the wells where the spheroids are settled. Slowly aspirate half the supernatant from each well without disturbing the spheroids, then gently replace with same amount of pre-warmed fresh media by dispensing along the well wall to avoid breaking the spheroids apart. For best results, avoid pipetting the whole amount of medium at once from the well, instead, perform repeated half media changes such that the majority of the media in the well has been replaced.
In order to transfer spheroids from the microwells, it is recommended that special pipette tips of large diameters (wide bore tips), such as the Finntip™ wide orifice pipette tips be used to accommodate the diameter of the spheroids to prevent damage.
Q5: I use cell viability assays for my monolayer cultures to assess culture health. Can I do this with spheroid cultures?
The ability to monitor the viability of the spheroid cultures is critical but can be difficult to achieve. Most commercially available cellular dyes and probes (i.e. PrestoBlue™ HS and alamarBlue HS) used to assess cell viability were developed and validated for 2D monolayer cultures, so caution must be taken when applied to 3D spheroids. While the molecular mechanisms associated with the functionality of these reagents remain the same, to overcome the thicker and denser nature of the 3D cellular spheroids, 3D protocols must be optimized to allow for better penetration by the reagents. Table 1 shows some commonly used cell viability reagents and the protocol adjustments required when used for 3D spheroids.
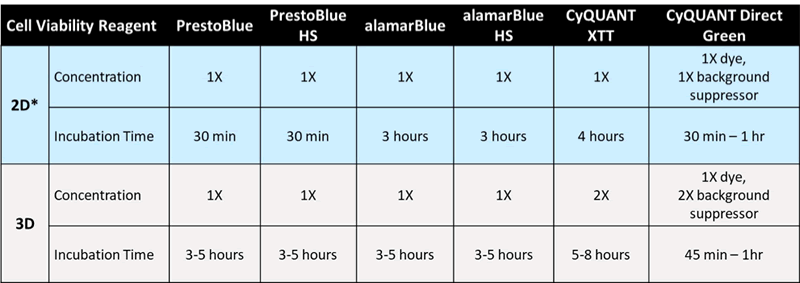
Table 1. Optimized incubation conditions of commonly used cell viability reagents on 3D cell spheroids
When adding the reagent, be sure not to penetrate the spheroid. Additionally, for tighter and more compact 3D structures, rotating more often during the incubation time is recommended but not required.
Q6: What modifications do I need to make in order to successfully perform immunostaining on my spheroids?
As with the cell viability assays, when looking to perform immunostaining on a 3D structure, protocol optimization may be required compared to the 2D monolayer equivalent. In general, the larger and tighter the spheroid, the longer and more complex it will be for complete staining to occur. Table 2 shows some commonly used reagents and the protocol adjustments required when assaying cellular functions in 3D spheroids.
| Cellular function | Apoptosis | Mitochondria Health | |
|---|---|---|---|
| Detection Reagent | Cell Event Green Caspase 3/7 | MitoTracker Orange | |
| 2D* | Concentration | 1X | 1X |
| Incubation Time | 30 min | 30 min | |
| 3D | Concentration | 1/3 X | 2X |
| Incubation Time | 2 hr | 1 hr | |
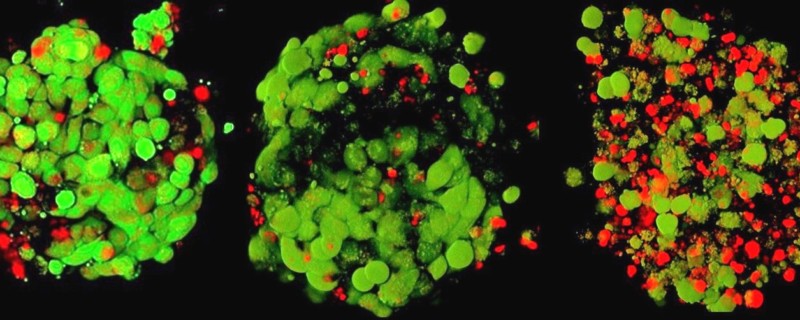
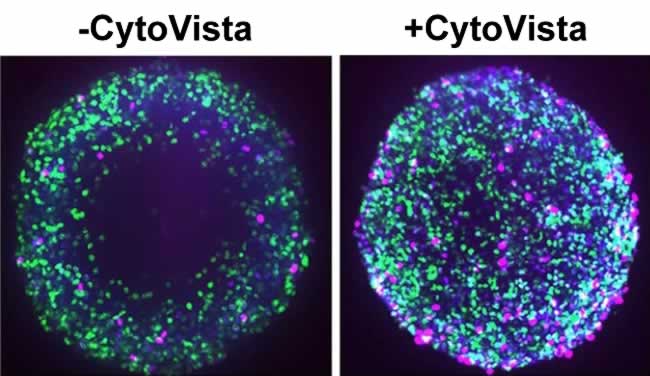
For cellular imaging in 3D spheroids, tissue clearing reagents such as the Invitrogen™ CytoVista™ 3D Cell Culture Clearing/Staining Kit improves staining reagent penetration to the core of the spheroids, enhancing the quality and the resolution of the image while retaining cell morphology (Figure 3). This clearing process enables sharp and bright 3D fluorescent imaging for depths up to 1000 µm and is compatible with most fluorophores. For protocols where cells are required to be fixed and permeabilized, the addition of 5% DMSO to the antibody staining solution can help reduce background. Additionally, more stringent wash steps with ½ volume change can help reduce the possible background issues related to the 3D culture imaging analysis.
Despite challenges that have previously limited broader use, new technologies for spheroid formation, culture, and analysis have made 3D spheroid systems a mainstream in vitro tool providing more predictive readouts than standard 2D cultures for many applications. Success in culturing spheroids relies on reliable tools such as the Nunclon™ Sphera™ plates, which have proven to produce spheroids that are uniform in size easily and efficiently, essential to generating meaningful and reproducible experimental results.
For more information, please visit https://www.thermofisher.com/sphera