Xeno-free media supplement for Cell Therapy Manufacturing
Cell-based therapy has demonstrated significant promise in the potential to treat some of the most challenging diseases. The recent FDA approvals of Novartis’ Kymriah and Kite Pharma’s Yescarta demonstrate the tremendous benefits this industry has to offer. As more and more cell therapies progress towards commercialization, safe and efficient manufacturing becomes critically important. Because the cell is the therapy, materials and methods used to culture the cells are a key focus of regulators. It is easy to become inundated by the plethora of reagents available on the market and risk mitigation strategies are becoming differentiators. Several companies have looked to serum-free culture in an attempt to remove the variability associated with using animal source sera. However, the ex vivo manipulation necessary for Cell Therapy manufacturing creates a more challenging system for the cells to survive in and thus they have a tendency to struggle in serum free conditions. If the cells aren’t growing well, then manufacturing can’t meet the efficiency or timelines required to create a viable product.
A growing trend is the use of xeno-free media or media supplements to provide cells with the nutrients they crave, while providing regulatory advantages over FBS. Nucleus Biologics recently launched a xeno-free media supplement designed for Cell Therapy that can be used in place of FBS or serum free media (SFM) supplements. The new product, called Physiologix™ XF hGFC, works much like FBS in that it can be added to basal media like RPMI 1640 or DMEM/F12 with little optimization required for good results. Nucleus Biologics has demonstrated that it can successfully replace FBS or provide better proliferation over serum-free media even in challenging cell lines such as mesenchymal stem cells and T cells.
I recently spoke with Alyssa Master, Ph.D., Senior Manager of Science and Applications, of Nucleus Biologics about Physiologix™ and have included some of the highlights of our interview here.
Brandy: What is Physiologix?
Alyssa: It is a human growth factor concentrate media supplement that is highly potent and xeno-free. It is sourced from transfusion grade donor material and processed through a proprietary manufacturing system into a highly physiologically relevant media supplement for stem cells, T Cells and other human cell lines.
Brandy: What would you identify as the key benefits of the new product?
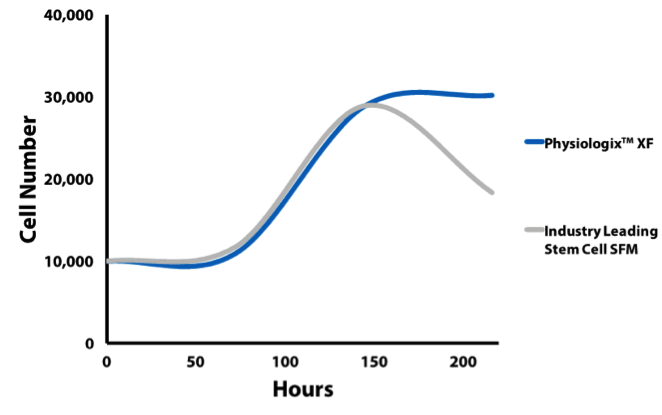
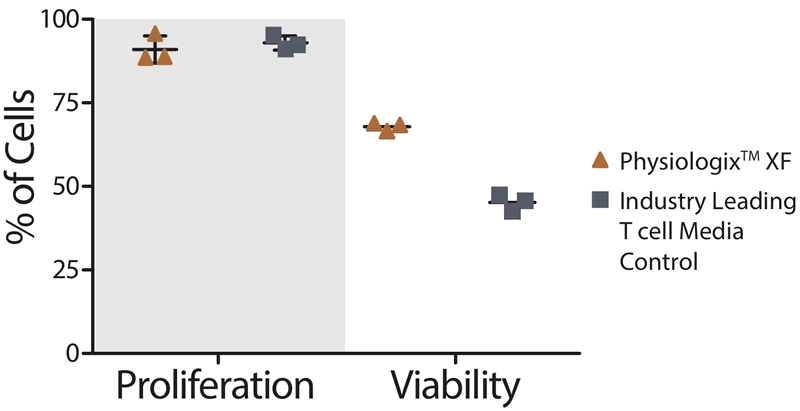
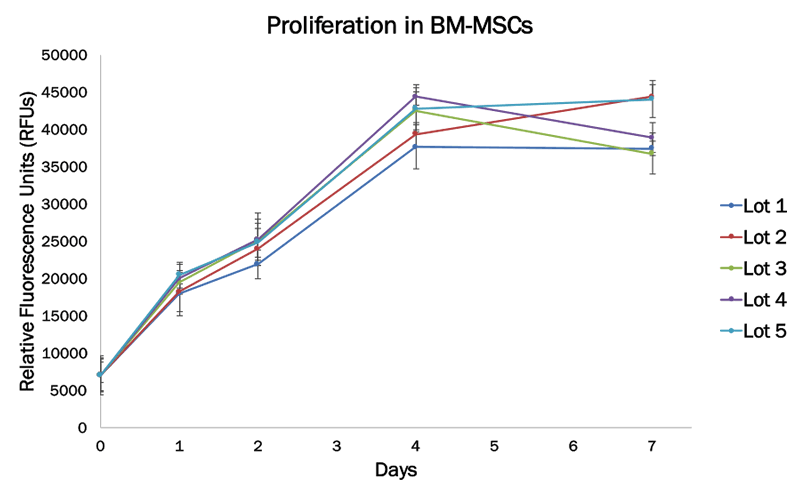
Alyssa: It is an exciting product because of how it has performed across multiple labs and cell lines. It can be added at a 2% concentration to basal media and we have seen significant improvements in cell proliferation over serum-free media even without optimization. This has proven true in the more finicky cell lines as well, for example CD4+ T Cells and Mesenchymal Stem Cells (Figure 1 and 2). We anticipate that with a bit of optimization and the addition of common supplements such as glutamine for some cell lines that the proliferation and viability will be even higher.
Proliferation data with bone marrow derived mesenchymal stem cells
CD4+ T cells Proliferation and Viability
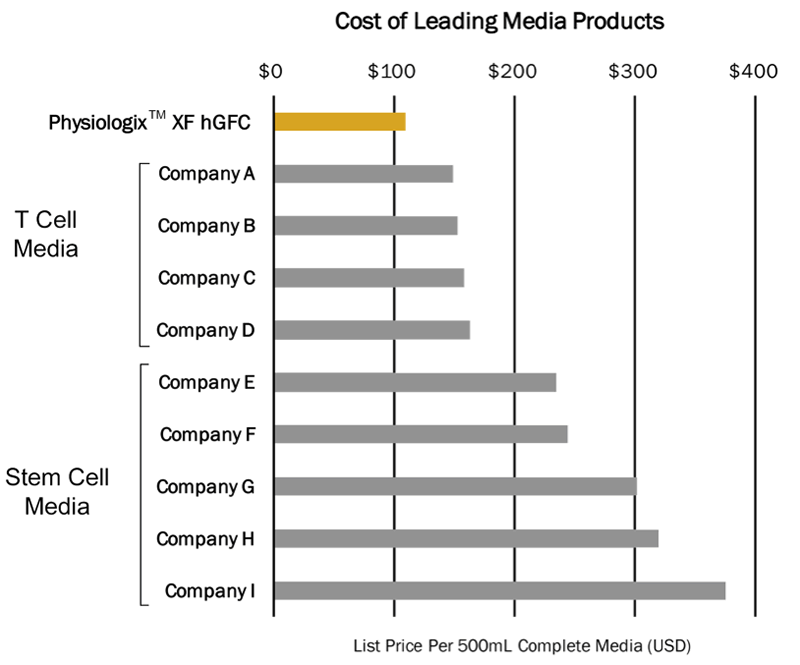
Another important benefit is that since it can be added to standard basal media, which is relatively inexpensive, the overall manufacturing cost is significantly less expensive when compared to commercially available pre-formulated serum-free media (Figure 3).
Brandy: What is most important to bioproducers?
Alyssa: When we sought early customer feedback we heard that with serum-free or chemically defined media it was sometimes difficult to achieve the culture consistency and reproducibility they wanted. It was key that Physiologix have a process that yielded demonstrable consistency across production lots. We think we have achieved that and now have the testing to prove it (Figure 4).
Brandy: What does the term xeno-free mean as it is sometimes used differently in the industry.
Alyssa: To Nucleus Biologics Xeno-Free means that is free from components that are of a different origin than the cell line being grown. Physiologix is extracted from transfusion grade human blood and free of any animal products. Also, there are no anticoagulants or other animal origin additives that are necessary to add to the final media to culture the cells. In short the most physiological system for human cells.
To learn more, please contact Alyssa Master (amaster@nucleusbiologics.com) or Steve Orzell (sorzell@nucleusbiologics.com ) at Nucleus Biologics.